Advances in Diabetes & Endocrinology
Download PDF
Research Article
Soluset Insulin Solution Treatment Algorithm (SISTA) for Hyperglycaemic Emergencies among Adults in Low Income Countries
Taoreed Azeez*
Department of Medicine, University College Hospital, Nigeria
*Address for Correspondence: Taoreed Azeez, Endocrinology, Metabolism and Diabetes Unit, Department of Medicine, University College Hospital, Ibadan, Nigeria
Submission: 28 August, 2020;
Accepted: 28 September, 2020;
Published: 01 October, 2020
Copyright: © 2020 Azeez T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Prevalence of diabetes mellitus is rising dramatically in low income
countries. Hyperglycaemic emergencies are among the commonest
medical emergencies in these countries. Managing these emergencies
is faced with multiple challenges. Intravenous insulin is the preferred
modality of administering insulin in these patients. Insulin pumps are the
ideal means of administering insulin but these are unaffordable and
relatively unavailable in the low income countries.
Administering insulin via the intravenous giving set is a common
modality of insulin therapy in the developing countries. This is associated
with wastages, discomfort for the patient and the insufficient nursing
staff. Wide fluctuations in glucose patter is a common finding in
these settings because the intravenous fluid giving set cannot be
finely regulated. Soluset is a volumetric cylinder used commonly in
Paediatrics but rarely used in Adult Medicine. It gives advantages
such as the ability to fine tune the rate of administering intravenous
medications.
Soluset Insulin Solution Treatment Algorithm (SISTA) is a proposed
modality to solve the problems of intravenous insulin administration in
adults especially in low income countries. It is readily available in low
income countries. It is also affordable. It gives the chance of fine tuning
insulin administration to optimize glycaemic control. The nursing staff
are already familiar with soluset and it does not require any special
training to use. It combines some of the advantages of insulin pump
with the advantages of insulin infusion with intravenous fluid giving set.
It is more affordable in low income countries compared with insulin
pumps. It also prevents wastages and wide glycaemic fluctuations
associated with intravenous insulin administration via the intravenous
fluid giving set, which is what is most commonly used in low resource
settings.
Keywords
Soluset insulin solution; SISTA; Treatment of hyperglycaemic emergencies; Hyperglycaemic emergencies in low income countries
Background
Diabetes mellitus is a heterogeneous group of metabolic disorders
characterized by chronic hyperglycaemia which results from a
deficiency in insulin secretion and/or action [1]. The majority of
people living with diabetes in the world are living in low income
countries and the prevalence of diabetes is increasing dramatically
in these countries [2]. According to the world bank, in 2019, low
income counties are those countries whose gross national income
per capita is less than $1025 [3]. Due to inadequate resources,
health care financing in low income countries is suboptimal and this
impairs health care delivery in these countries [4]. The facilities and
infrastructure for managing in-patients available in the developed
countries are largely unaffordable in low income countries and there
is a need for adapting available technology with the aim of getting the
best care with minimal cost.
Insulin is a peptide hormone that can be used as a drug in the
treatment of hyperglycaemia. Frederick G Banting, Charles H Best
and JJR Macleod were credited with the discovery of insulin following
their works at the University of Toronto [5]. In documented
literature, the first patient to be treated with insulin was a 14 year
old boy, Leonard Thompson, who was a diabetic patient at the
Toronto General Hospital [5]. Eli Lilly and Company was the first
pharmaceutical company to produce insulin in commercial quantities
[5]. This changed the history of management of diabetes from being
a death sentence to a disease that can be managed through adequate
replacement of the deficient hormone.
The insulins that were first administered to diabetic patients
were derived from animals. These insulins were extracted from
the pancreas of animals such as pigs (porcine insulin) and cattle
(bovine insulin) and are later purified through meticulous industrial
processes to prevent reactions to the animal insulins by the patients
on insulin therapy. This went on until the 1980s. Through genetic
engineering and intensive researches, Eli Lilly Corporation massproduced
human insulin [5]. This was a paradigm shift in insulin
pharmacotherapy. Organisms such as Escherichia coli and yeasts
are being used to grow human insulin through the process of
Deoxyribonucleic (DNA) technology [5]. The manufactured insulins
are subjected to purification processes such as high performance
liquid chromatography, gel filtration and x-ray crystallography
to ensure quality control. By the mid 1990’s, researchers started
working on the modification of the amino acid sequencing coded by
the insulin gene so as to produce insulin with better pharmacokinetic
and pharmacodynamic properties. These insulins are called analogue
insulins. Examples of the analogue insulins include rapid actin
insulins such as (lispro, aspart and glulisine), long acting insulins
(such as detemir and glargine) and ultra-long acting insulins (such
as degludec).
Insulin can be administered subcutaneously, intramuscularly or
intravenously. Oral insulins are still under intense research while
inhaled insulins have not received clinical patronization compared with the initial enthusiasm that welcomed their discovery. This is due
to issues of efficacy and safety. Nasal, buccal, transdermal, rectal and
transperitoneal insulin administration have all been documented in
the literature but they have no clinical relevance as far as guidelines
on diabetes management are concerned [6]. The subcutaneous route
is the commonest route of administration of insulin. The modalities
of subcutaneous insulin injections include the use of insulin syringe,
insulin pens and Continuous Subcutaneous Insulin Infusion (CSII)
[6].
Insulin pumps joined the diabetes therapy armamentarium in
the 1970s [6]. It comes in various sizes and models. In the developed
countries, insulin therapy via insulin pumps is gradually becoming
the standard of care, especially in type 1 diabetes [7]. While older
generation insulin pumps are manually activated by the patient
to deliver insulin, especially insulin boluses, the newer generation
insulin pumps are mostly automated. With modern technology,
data on glucose levels and insulin administered can be retrieved
and reviewed by the patients and the physicians [6]. Randomized
controlled trials and meta-analyses have reported the clinical
advantages of insulin pumps compared with Multiple Daily Insulin
Injections (MDII), in terms of glycaemic control [8]. Insulin pumps
are however unaffordable and unavailable to diabetic patients in
low income countries [8]. An average cost of insulin pump is about
$10 000 and consumables such as reservoirs and infusion sets cost
$25 - $30 per month. A systematic analysis reported the total cost
of medications for the treatment of diabetes per year per capita in
low income countries is about $15 - $500 [9]. Clearly, this shows that
insulin pumps are not affordable in low income countries.
Overview of hyperglycemic emergency:
In patients with diabetes mellitus, either previously or newly
diagnosed, Hyperglycaemic Hyperosmolal State (HHS) and Diabetic
Ketoacidosis (DKA) are forms of hyperglycaemic emergencies that
have been documented in them. Both can occur in all forms of
diabetes but DKA occurs mostly in type 1 diabetes while HHS occurs
mostly in type 2 diabetes [10]. Also, while DKA tends to occur in
younger adults, HHS is commoner in middle-aged and old people.
Mortality rate in DKA was close to 100% before the discovery and
clinical usage of insulin [10]. Following the use of insulins, mortality
dropped rapidly to about 60% and this has progressively reduced
over the century to about 2% in DKA and about 5-15% in HHS [11].
In low income countries, the mortality rates from hyperglycaemic
emergencies is higher compared with the developed countries [11].Common presenting symptoms in patients with hyperglycaemic
emergency include polyuria, polydipsia, weight loss, weakness,
nausea, vomiting, abdominal pain and altered sensorium [10]. The
most documented signs in hyperglycaemic emergencies include
altered consciousness, dehydration, hypothermia, tachycardia,
hypotension and tachypnoea [10]. Kussmaul breathing and acetone
breath are peculiar to DKA [10]. The triad of laboratory findings in
DKA are hyperglycaemia, increased anion-gap metabolic acidosis
and hyperketonemia (or ketonuria) [10]. In HHS, the common
laboratory findings are severe hyperglycaemia (usually above 600mg/
dl), hyperosmolality and the absence of severe ketoacidosis [12].
Intravenous insulin therapy:
Hyperglycaemic crises are among the commonest reasons for
admissions to the general medical wards, high-dependency units and
the Intensive Care Units (ICU). Studies have shown that intravenous
insulin therapy controls hyperglycaemia more efficiently than any
other route of administering insulin and it is the recommended
route in most guidelines [13]. Improved glycaemic control has been
associated with improved clinical outcomes, in terms of morbidity
and mortality [12].In hyperglycaemic emergencies, critically ill patients, women in
labour, diabetic patients on Nil Per Oral (NPO) and perioperative
patients, intravenous insulin is the preferred route of administering
insulin [14]. This is because the rapid onset (within seconds) and short
duration (within 5-10 minutes) of soluble or rapidly acting insulin
makes it easier to match insulin dose to the glucose level in order to
achieve the glycaemic goals during the treatment of hyperglycaemic
emergencies [13].
The necessary resources that must be available to attain the
glycaemic targets when intravenous insulin therapy is used are
insulins, trained nurses, glucometers and institutional protocols.
Hypoglycaemia is the commonest drawback of intravenous insulin
therapy and this is of great significance in critically ill patients who
may not manifest the classical clinical features of hypoglycaemia.
Intravenous insulin therapy and monitoring are supposed to be
nurse-driven with valuable input from the physician. Intravenous
insulin therapy is often implemented using either insulin pump
(usually in developed countries) or intravenous fluid giving set in
form of glucose-potassium-insulin infusion (usually in low resource
settings) but both have significant demerits.
Insulin therapy using intravenous pumps:
Insulin pump is a medical device that is programmed to deliver
insulin at a controlled rate. The various intravenous pumps that are
available include gravity-infusion device, volumetric pumps and
syringe pumps. Syringe pumps are the most commonly used type
[13]. The intravenous pump is relatively accurate in the delivery of
the required insulin doses. Some pumps have in-built batteries so
that they can function during power outage. While insulin is being
administered via the pump, glucose infusion is given via the fluid
giving set to prevent hypoglycaemia.The pumps are built with safety measures such as the ability to
detect air in the tubing. Insulin pumps have some disadvantages. In
low resource settings, cost and availability are the main issues. Also,
there is a need for special training in the usage of the pumps. Repair
and replacement of malfunctioning parts may be difficult in low
resource settings. Additionally, in low resource settings where power
supply is erratic, use of intravenous pumps may be a challenge. Also,
an abnormality with either the pump tubing or the fluid giving set
tubing results in hyperglycemia or hypoglycemia respectively.
Intravenous insulin therapy using the Glucose-Potassium-Insulin (GKI) infusion:
GKI infusion is a solution of a dextrose fluid (5% dextrose water
for example), intravenous potassium and insulin (usually soluble
insulin but rapid acting insulins may also be used). It is also known
as the Alberti regimen [15]. The amount of each constituent depends on the glucose level, glycaemic target and potassium level. This allows
insulin, electrolyte and fluid to be given together. Blood glucose
level is checked hourly. Some of the challenges with the use of GKI
infusion include the fact that it is difficult to regulate and it exposes
the patient to wide fluctuation of glucose levels. It is also wasteful
because whenever the checked blood glucose is not up to the expected
level, the on-going fluid has to be discarded and a new solution
reconstituted. For patients who pay out of pocket, which is a common
thing in low resource settings, this is of enormous financial impact.
GKI involves administering insulin mixed with fluids and this may
become a significant clinical challenge in people with fluid retaining
co-morbidities such as heart failure o renal failure. Hypokalemia is
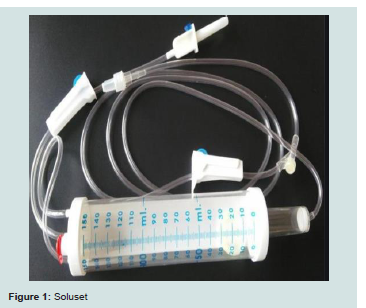
also a common finding with the use of GKI.Soluset
Solusets are special volumetric cylinders often used for intravenous administration of medications, especially in Paediatrics [16]. A soluset is shown in Figure 1 below.
Principles of intravenous insulin therapy using soluset
With soluset intravenous infusion, 60 drops = 1 ml.
The formula that underlies intravenous drug infusions is given
below.
Amount of insulin given (Units) = Concentration (Units/ml) X volume of the solution given (ml)
In a hyperglycaemic emergency, the patient typically needs
two Intravenous (IV) accesses. One access is connected to the GKI
infusion for intravenous insulin therapy and the other access is
connected to IV fluid (usually 0.9 or 0.45 saline) for rehydration of
the patient. With soluset, only one IV access is needed because the
second IV giving set for rehydration is connected to the ‘Y’ junction
of the soluset tubing. This translates to better comfort for the patient
and lower risk of thrombophlebitis.
During resuscitation of a patient with hyperglycaemic emergency, capillary glucose is monitored regularly, usually hourly. If there is a need to increase or reduce the rate of insulin infusion, the GKI infusion has to be discarded and a new one reconstituted at every instance, causing a lot of wastages. With soluset insulin infusion however, the rate of infusion, in terms of number of drops per minute, is the only thing that is changed while the ongoing infusion continues.
How to adjust soluset insulin infusion:
The first thing is to determine the amount of insulin to be given per time. For example, if 0.1 Unit/kg/hr of insulin is to be administered via soluset, how that can be achieved.A pint of IV Normal saline (500 ml) is used to prepare the insulin solution. 50 units of soluble insulin is injected into the 500 ml of normal saline.
The concentration of insulin in the normal saline is calculated as follows:
C- Concentration of insulin in the normal saline (U/ml)
n- Amount of insulin added into the insulin = 50 units (as given above)
V- Volume of the normal saline=500ml
Kg- weight in kilogram
hr- time in hours
Therefore
C = 0.1 U/ml
The concentration of insulin in a 500ml of normal saline when 50 units of soluble insulin is added into it is 0.1 U/ml.
The normal saline is then connected via a port to the cylinder
of the soluset and some insulin solution is allowed to flow into the
cylinder (usually 150 ml at a time, as that is the maximum capacity of
the soluset cylinder).
So, in order to administer 0.1 U/Kg/hr of insulin to the patient
using the solution constituted above, the next thing is to determine
the weight of the patient. 70 kg is adopted for this illustration.
The amount (A) of insulin to be administered per hour to this
patient is calculated below:
A = 0.1 X 70 X 1
A= 7 U/hr
So, it has been determined the 7 Units of insulin will be given per hour, so the next thing is to determine the volume of the insulin solution (constituted above) that will be administered via the soluset.
Therefore V =
Where V-volume of insulin solution to be administered per hour
A - Amount of insulin to be administered per hour = 7 U/hr (as determined above)
C - Concentration of insulin in the insulin solution = 0.1 U/hr
V = 7/0.1
V = 70 ml/hr
So, in order to give 0.1 U/kg/hr of soluble insulin to a 70 kg man,
using insulin solution containing 50 Units of soluble insulin inside
500 ml of normal saline, the patient will need 70ml/hr of the solution.
In order to give 70ml/hr of the insulin solution administered via soluset, the number of drops per minute has to be determined.
Using a soluset, 6 drops = 1 ml (rule of thumb)
Therefore, 70 ml= (70 x 60) drops = 4200 drops
So, 4200 drops are to be given per hour
Which means 4200 drops are to be given per 60 minutes
There 70 drops are to be given per minute.
In summary, in order to give 0.1 U/kg/hr of soluble insulin to a 70 kg man, using insulin solution containing 50 Units of soluble insulin inside 500 ml of normal saline, the patient will need 70 ml/hr of the solution which translates to 70 drops per minute when given via soluset. So, adjusting the dose of insulin given is simply by changing the number of drops per minute without discarding the ongoing infusion.
Soluset Insulin Solution Treatment Algorithm (SISTA) for hyperglycaemic emergencies using the American Diabetes Association (ADA) guidelines:
According to ADA, there are four main goals of therapy in the
management of hyperglycaemic emergency. These include circulatory
volume restoration, gradual reduction of osmolality and glucose,
addressing electrolyte derangement and treating co-morbidities [17].Fluid therapy with SISTA:
Fluid resuscitation is central to the management of hyperglycaemic crisis [17]. The fluid deficit in DKA is about 6-8 L while for HHS, it is about 8-10 L. With SISTA, an IV normal saline is connected via the IV fluid giving set to the ‘Y’ junction of the soluset tubings. About 1- 1.5 L of normal saline is given in the first hour, then another 1-1.5L in the next 2 hours. Thereafter, 1-1.5 L is given over the next 4 hours. If the patient is making adequate urine and he/she is not hypotensive
and not hyponatraemic, the fluid may be changed to 0.45% saline to prevent hypernatremia. If 0.45% saline is not available, as it is the case in many low resource settings, 5% Dextrose in 0.45% saline is constituted, under sterile technique, by mixing 250 ml of 10% Dextrose water with 250 ml of normal saline. 5% Dextrose in 0.45% saline can be used but insulin flow rate has to be adjusted. The target rate of drop of osmolality is about 3mosm/kg/hr while the target rate of drop of sodium is 0.5 mmol/L.Potassium therapy with SISTA:
After the first hour of IV fluid, IV Potassium Chloride (KCl) may be added into the fluid, depending on the serum potassium. If serum potassium is above 5mmol/L, potassium should not be added. If potassium is 3.5-5 mmol/L, add 20 mmol of IV KCl into 1L of fluid. If serum potassium is less than 3.5 mmol/l, add 40 mmol of IV KCl into IL of fluid and wait for the potassium to rise to at least 3.3 L/min before commencing insulin therapy.Insulin therapy with SISTA:
Continuous infusion of insulin via the soluset at 0.1 U/kg /hr is commenced. How to give this via soluset has been illustrated above. Random Blood Glucose (RBG) is monitored hourly and the target rate of drop of glucose is 50-70 mg/dl/hr. Too rapid drop may precipitate cerebral oedema (especially in children and adolescent) and hypoglycaemia. If the rate of drop of blood glucose is less than desired, the insulin rate may be doubled to 0.2 U/Kg/hr. Similarly, if the rate of drop of the blood glucose is higher than desired, the rate of drop may be halved to 0.05 U/kg/hr.When RBG is less than 250 mg/dl, the rate of insulin therapy is
halved into 0.05 U/Kg/hr. Also, the fluid that is connected to the ‘Y’
junction of the soluset is changed into 5% Dextrose water in 0.45%
normal saline. How to constitute 5%Dextrose water in 0.45% normal
saline is explained above.
Advantages of SISTA and the relevance in low resource settings:
Intravenous drug treatment with soluset is not new. It is a
common practice in Paediatrics, especially in Neonatology. However,
there is no documentation of its usage in hyperglycaemic emergencies
among adults. In a hyperglycaemic emergency, intravenous insulin
is the preferred route of administering insulin. The two commonly
documented avenues of doing this is through insulin pumps and
insulin infusion using IV fluid giving set.Insulin pump is expensive and it is not readily available in low-resource settings. Intravenous insulin infusion via the IV fluid giving set is prone to wide fluctuations in glycaemic control. The monitoring may be suboptimal in low income countries where the nurses: patients ratio is very low. Poor monitoring is associated with suboptimal glycaemic control. It is also prone to wastages.
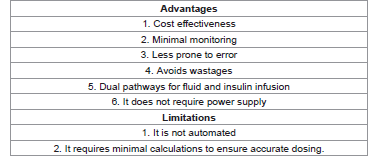
The Soluset Insulin Solution Treatment Algorithm (SISTA) is being proposed as an effective alternative for intravenous insulin therapy, especially in low income countries. It mitigates against wastages because adjusting insulin dose does not require discarding the on-going fluid and reconstituting another one. Rather, adjusting the number of drops per minute is sufficient to bring about the desired change in the insulin dose. The soluset is readily available in low resource settings. It is relatively cheap and it is easy to use by the nursing staff. It is relatively easy to calculate the number of drops per minute, and it is less prone to wide fluctuations in glycemic control. It has a ‘Y’ junction where the giving set for the rehydrating fluid is connected. This is more comfortable for the patient and the risk of thrombophlebitis is reduced. A clinical trial is being proposed to document the efficacy of SISTA. Ethical approval and feasibility studies are presently ongoing to facilitate the trial. The summarizes the advantages and the limitations of adopting SISTA (Table 1).
Conclusion
Diabetes mellitus is most prevalent in the low income countries.
Similarly, episodes of hyperglycaemic emergencies are high in
these counties. Intravenous insulin therapy is the preferred route of
administration. Insulin pumps are largely not affordable and available
in low resource settings. Intravenous therapy using GKI is also faced
with its own challenges such as wastages and wide fluctuations in
glycaemic pattern. Soluset Insulin Solution Treatment Algorithm
(SISTA) is hereby being proposed as an effective alternative for
intravenous insulin therapy during hyperglycaemic emergencies in
low income countries. The advantages include the affordability, the
widespread availability, the comfort for the patient and the ease of
monitoring the treatment. It is an outcome of adapting available
technology to solve clinical problems in resource deficient areas.
There are however needs for clinical trials to validate the effectiveness
of this novel approach of administering insulin, especially in low
income countries.