Journal of Bioelectronics and Nanotechnology
Download PDF
*Address for Correspondence: Mohit Rawat, Department of Nanotechnology, Sri Guru Granth Sahib World University, Fatehgarh Sahib 140406, Punjab, India, Tel: +918872108007, E-mail: mohitnano.nit@gmail.com
Citation: Singh J, Kaur G, Rawat M. A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications. J Bioelectron Nanotechnol 2016;1(1): 9.
Copyright © 2016 Singh J et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Bioelectronics and Nanotechnology | Volume: 1, Issue: 1
Submission: 19 May, 2016 | Accepted: 23 June, 2016 | Published: 27 June, 2016
Reviewed & Approved by: Dr. Khalid Mujasam Batoo, King Abdullah Institute for Nanotechnology, King Saud University, Saudi Arabia
For the formation of CuO nanoparticles from Cu2O precipitant, the following reaction mechanism can be formulated as represented as:
Mousa MK proposed fast, inexpensive and simple quick precipitation method and control the size with different temperature; 65,75 and 85 °C. By increasing temperature leads to decrease of nanoparticles size [15]. The sizes of CuO-TOAB stabilized nanoparticles are smaller than CuO non stabilized nanoparticles that prepared at the same temperature. X-Ray diffract to gram peaks showed that all obtained CuO NPs have monoclinic structure. Studying the effect of different concentrations of NaOH reducing agent and TOAB surfactant on the CuO nanoparticles size and morphology and antibacterial activity of CuO nanoparticles with other surfactants can be considered as future work (Figure 11).
Nasibulin AG, et al. [16] studied the thermal decomposition of copper acetylacetonate vapor using a vertical flow reactor at ambient nitrogen pressure for the synthesis of Crystalline nanometersizedcopper (I) oxide particle. They performed experiments in the precursor vapor pressure range of Pprec= 0.06 to 44 Pa at furnace temperatures of 431.5 °C, 596.0 °C, and 705.0 °C. Crystalline product of the decomposition depended on the precursor vapor pressure. The formation of copper (I) oxide particles occurs due to the surface reaction of the decomposition products (mainly carbon dioxide). They proposed a model to build a semi empirical phase diagram of the precursor decomposition products (Figure 12).
Zhu H, et al. proposed a wet chemical method to synthesize stable CuO nanofluids in a large-scale [17]. Different copper salts resulted in different particle morphologies. Size and shape of clusters of primary nanoparticles are affected by concentration of copper acetate and reaction time. By changing the synthesis parameters nanofluids with different microstructures could be obtained. The thermal conductivity of CuO nanofluids increased with the increase of particle loading(Figure 13).
Kannaki K, et al. proposed the hydrothermal route for synthesis of copper oxide nano particles [18]. It is confirmed by XRD analysis. The UV-Visible study shows the radiation in red region can be applicable for fabrication of optoelectronic devices. The FTIR analysis reveals the characterization peaks for CuO stretching. The adsorption of ammonia is confirmed by FTIR spectra. The spherical Morphology is confirmed by SEM/EDS (Figure 14). This method may be suitable for large scale production of CuO nanoparticles for practical applications.
Darezereshki E, et al. spherical CuO nanoparticles with mean diameter of 170±5 nm have been prepared by a thermal decomposition method [19]. This method does not require organic solvents, expensive raw materials and complicated equipment. So, it is concluded that the presented method is superior to the other methods for the synthesis of CuO nanoparticles from dilute CuSO4 solution (Figure 15).
Srivastava S, et al. Cupric oxide (CuO) nanoparticles were prepared by the chemical route by calcinations at a higher temperature from 300 °C to 400 °C [20]. For the comparison transmission electron microscopy (TEM) and x-ray diffraction (XRD) measurements were made through JCPDS. There is good agreement between data produced by spectroscopy and the microscopic measurements.
Nithya K, et al. Copper oxide nanoparticles were prepared by modified sol-gel technique using sodium dodecyl sulphate as a surfactant [22]. Effect of calcination temperature on particle size, band-gap, crystallinity and morphology of the nanoparticles were studied with the help of particle size analysis, UV-Spectroscopy, powder X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) studies (Figure 17). Prepared nanoparticles will be tested for their activity towards gas sensing.
Karthik AD, et al. the present investigation reports, the novel synthesis of Copper and Copper oxide nanoparticles using Chemical reduction method and its physicochemical characterization [23]. The nanoparticles have been prepared using Copper (II) succinate as precursor. Copper nanoparticles are initially formed and subsequently oxidized to copper oxide. As reported the nanoparticles were characterized by UV-visible spectroscopy, Fourier transform infra-red spectroscopy (FTIR), X-ray diffraction measurements (XRD) and scanning electron microscopy (SEM). SEM analysis exhibited nanoparticles with an average diameter of about 45 nm (Figure 18). XRD analysis revealed broad pattern of fcc crystal structure of copper metal and cubic cuprites structure for Cu2O. The antimicrobial properties of copper nanoparticles were investigated using Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. The bactericidal effects of copper nanoparticles were studied based on diameter of inhibition zone in disk diffusion tests of nanoparticles dispersed in batch cultures. The copper nanoparticles showed excellent activity against Escherichia coli and Staphylococcus aureus, with excellent inhibition zones of 14 mm and 10 mm, respectively. Bacterial sensitivity to nanoparticles was found to vary depending on the microbial species.
Shah MA, et al. temperature and pressure, both of which can affect the super saturation and nucleation are responsible for solvents properties [24]. In this study, we use water as solvent under supercritical conditions and report copper (Cu) and (Cu2O) nanoparticles of size ranging from 9 nm to 60 nm. This synthetic technique has the following advantages: Firstly, it is one step synthesis approach, making it easy to control the growth kinetics. Secondly, the synthesis needs no sophisticated equipment. Thirdly, the approach is non-toxic without producing hazardous waste as water is being used as solvent as well as source of oxygen. Forth, it is a surfactant free synthesis and has bright prospects.
Rani R, et al. Purewal Monoclinic CuO nanoparticles were synthesized by reverse micelle technique [26]. Size and morphology were characterized by using XRD, and TEM techniques. FTIR technique was used to ensure the bonding between metal and oxygen. Antibacterial activity of CuO nanoparticles were tested against bacterial strains K. pneumoniae, S. typhimurium, and E. aerogenes using the agar diffusion method. Minimum inhibitory concentration of these three gram-negative bacterial strains K. pneumoniae, S. typhimurium, and E. aero genes were found to be 0.55, 0.15, and 0.30 μg/ml, respectively (Figure 21).
Devi HS, et al. this work is to report an environmental benign route for the fabrication of copper oxide nanoparticles using Centella asiatica (L.) leaves extracts at room temperature [28]. This method is completely a green method, free from toxic and harmful solvent. Copper oxide particles such prepared are in nanoscale and there morphology and size are characterized using SEM, UV-Visible spectroscopy, IR spectroscopy and EDX. Copper oxide nanoparticles synthesized by this method can be used for the photo catalytic degradation of methyl orange. These nanoparticles can reduce methyl orange to its leuco form in aqueous medium in the absent of reducing agents. It is more economy as compare to other methods. This catalytic effect of copper oxide nanoparticles can be contributed to its small size. Nanoparticles have many active sites as compared to the bulk materials because of its large surface to volume ratio. Copper oxide nanoparticles such prepared has good catalytic properties.
Wua R, et al. In this paper, highly dispersed copper oxide (CuO) nanoparticles with different size and morphology have been successfully prepared by a quick-precipitation method in DMACwater mixed solvent [32]. The as-prepared CuO nanoparticles were characterized by high resolution transmission electron microscopy (HRTEM) and x-ray diffraction (XRD) (Figure 26). Several kinds of CuO particles with different morphology were obtained including panicle-shaped, spherical-shaped, spindly-shaped and rod-shaped CuO particles. The effects of processing parameters on the size and shape of CuO particles such as volume ratio of DMAC/water, adding temperature of NaOH and molar ratio of were investigated. The nucleation and growth kinetic of the resulting CuO particles were also discussed.
Deng D, et al. copper nanoparticles are alternative for silver and gold nanoparticles which are currently used in inkjet printing of conductive patterns because of the low price and high electrical conductivity [33]. However, the serious impediment to using copper nanoparticles for conductive inks is their spontaneous oxidation. In the paper, the well-dispersed anti oxidative copper pastes were prepared by dispersing the nanoparticles in an ethanol solution of lactic acid, and then deposited on glass slides. The resistivity of conductive copper films was lAx10-5 n·cm after annealing at 200 °C for 30 min. It was experimentally proved that lactic acid could react with the copper oxides surrounding Cu to form copper carboxylate, which was then reduced to Cu after annealing at 200 °C under nitrogen atmosphere. Furthermore, the copper film after annealing at 200 °C for 30 min under nitrogen atmosphere showed anti oxidative characteristic (Figure 27).
Rout L, et al. this paper describes the CS cross coupling of thiols with iodobenzene in the presence of relatively inexpensive air stable CuO nanoparticles [36]. A variety of thiols could be cross-coupled with iodobenzene in this simple and efficient reaction to give the desired products in high yields.
Lo1 CH, et al. the optimal parameters are found for preparing nanofluid in our submerged arc nanoparticle synthesis system (SANSS) using a copper electrode [40]. A suspended copper oxide nanofluid is thus produced at the current of 8.5-10 A, voltage of 220 V, pulse duration of 12l s, and dielectric liquid temperature of 2 °C. The CuO nanoparticle are characterized by transmission electron microscopy (TEM), field emission scanning electron microscope (FESEM), X-ray diffraction (XRD), electron diffraction pattern (SAD) and electron spectroscopy for chemical analysis (ESCA). The equality volume spherical diameter of the obtained copper oxide particle is 49.1 nm, regular shape and narrow size distribution (Figure 30).
A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications
Jagpreet Singh, Gurjas Kaur and Mohit Rawat*
- Department of Nanotechnology, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab, India
*Address for Correspondence: Mohit Rawat, Department of Nanotechnology, Sri Guru Granth Sahib World University, Fatehgarh Sahib 140406, Punjab, India, Tel: +918872108007, E-mail: mohitnano.nit@gmail.com
Citation: Singh J, Kaur G, Rawat M. A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications. J Bioelectron Nanotechnol 2016;1(1): 9.
Copyright © 2016 Singh J et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Bioelectronics and Nanotechnology | Volume: 1, Issue: 1
Submission: 19 May, 2016 | Accepted: 23 June, 2016 | Published: 27 June, 2016
Reviewed & Approved by: Dr. Khalid Mujasam Batoo, King Abdullah Institute for Nanotechnology, King Saud University, Saudi Arabia
Abstract
In this paper, brief review for preparations of copper oxide nanoparticles by the different route are described. Since last few years, synthesis of nanoparticles has been attracted considerable attention. The metal oxides are important technology materials used as catalysts in chemical industries and in electronic and photonic devices. Due to the applications in advanced technologies, researchers have focused more on synthesis of CuO nanoparticles. Copper oxide nanoparticles appear as a brownish-black powder. They can be reduced to metallic copper when exposed to hydrogen or carbon monoxide under high temperature. Copper oxide nanoparticles are used in wide range of applications such as catalysis, gas sensors, magnetic storage media, batteries, solar energy transformer, semi conductors and field emission. CuO, as a P-type semiconductor exhibiting narrow band gap, have attracted great attention due to its potential application in nano devices such as electronic, optoelectronic and sensing. It has been widely used as powerful heterogeneous catalyst because of its high activity and selectivity in oxidation and reduction reactions.Keywords
Nanoparticles; Coppper; SemiconductorIntroduction
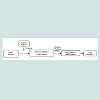
Copper oxide is a compound from two elements copper and oxygen, which are block d and block p elements in periodic table respectively. In a crystal copper ion is coordinated by four oxygen ions. Copper (Cu) and copper oxide (Cu2O) nanoparticles have attracted considerable attention because copper is one of the most important in modern technologies and is readily available [1]. There is increasing interest on copper nanoparticles due to their optical, catalytic, mechanical and electrical properties [2,3]. Copper oxide is widely used in the field of catalysis, superconductors, and ceramics as a kind of important inorganic materials. It can be used as a catalyst and catalyst support, as well as electrode active materials such as degradation of nitrous oxide with ammonia and oxidation of carbon monoxide, hydrocarbon and phenol in supercritical water [4]. Copper oxide nanoparticle is a powder soluble in dilute acid, NH4Cl, (NH4)2CO3, potassium cyanide solution, insoluble in water, and it dissolves slowly in alcohols, ammonia solution. It can be reduced to metallic copper when meets hydrogen or carbon monoxide under high temperature. CuO nanoparticle can also be used as burning rate catalyst in rocket propellant. Nano copper oxide shows superior catalytic activity and selectivity than that of the common copper oxide powder. The particle size of nanometre copper oxide is between 1-100 nm. Compared with the ordinary copper oxide, nano CuO has peculiar physical and chemical properties such as: surface effect, superiority of the quantum size effect, volume effect and macroscopic quantum tunnelling effect in magnetic, optical absorption, chemical activity and thermal resistance, catalysis, and the melting point. Nano copper oxide attracts more and more people’s attention, and become one of the most extensively used inorganic materials (Figure 1).Detailed Review
Mustafa G, et al. proposed the preparation of CuO nanoparticles of monoclinic structure and characterized by XRD analysis [5]. Due to irregular shapes ranges from 0.02 to 1 μm the nanoparticles provide a large surface area for the adsorption and used as adsorbent for the removal of MB dye. Adsorption, desorption, thermodynamic and kinetics studies were proceeded to determine the validity of process. It was estimated that inexpensive and cost effective materials can be prepared as adsorbents for the removal of dyes and metals (Figures 2a and 2b).For the formation of CuO nanoparticles from Cu2O precipitant, the following reaction mechanism can be formulated as represented as:
2CuSO4 + 2NH2OH.HCl + 6NaOH → Cu2O + N2 + 2NaCl + 2Na2SO4 + 7H2O (1)
Cu2O →CuO (2)Δ.
Suleiman M, et al. concluded that CuO nanoparticles can be synthesized by different ways and synthesis factors such as method, solvents, surfactants starting precursors and temperature are used to control the shape and size of desired nanoparticles [6]. Pulse wire explosion method is used for preparation and TEM is used to determine the particle size of nanoparticles (Figure 3).
Aparna Y, et al. demonstrated that CuO nanoparticles have potential applications for solar energy transfer, sensors, storage devices and in super conductors [7]. CuO nanoparticles act as a good catalyst in chemical reactions. They have prepared CuO nanoparticles by sol gel technique and concluded that XRD pattern revealed CuO nanoparticles have monoclinic structure. EDX analysis shows pure that CuO nanoparticles are pure and free from impurities. SEM shows good agglomeration of CuO nanoparticles.
Abboud Y, et al. proposed an eco-friendly and convenient method for the synthesis of CuO nanoparticles using brown alga (Bifurcaria bifurcata) extract [8]. No chemical reagent or surfactant template was required in this method, which consequently enables the bioprocess with the advantage of being environmental friendly. The developed nanoparticles were characterized by UV-VIS, TEM, XRD and FTIR measurements and showed good antibacterial activity. This biosynthesis technique can be a promising method for the preparation of other metals and metal oxide nanoparticles and can be valuable in environmental, biotechnological, pharmaceutical and medical applications (Figure 4).
Lanje AS, et al. studied that CuO nanoparticles are rectangular in shape with average size of 5-6 nm with monoclinic structure are synthesized by aqueous precipitation method [9]. The band-edge emission peak is found at 398 nm and green emission peak is found at 527 nm. The band-edge absorption is found to be at 355 nm (Figures 5 and 6).
Volanti DP, et al. proposed hydrothermal microwave method for the synthesis of CuO flower-nanostructures after thermal treatment at 393 K for 1 h [10]. The CuO flower-nanostructures with single phase were identified by XRD and micro-Raman techniques and present monoclinic lattice. The average diameter is of CuO flower nanostructures = 1.3 m. The obtained CuO flower-nanostructures are promising candidate for potential application in catalysis (Figure 7).
Sahay R, et al. fabricated highly crystalline CuO nanofibers with possible energy applications analyzed the effect of the dwelling time of the annealing cycle for the formation of the crystallite CuO nanofibers and observed with an increase in the dwelling time the crystallite size of CuO nanofibers decreased and results in the improved orientation of the CuO nanofibers [11]. The highly ordered morphology obtained at 12 h was employed for photo-catalytic study. A 25% increase in the current density was observed with the application of CuO as blocking layer.
Wongpisutpaisana N, et al. proposed that well defined CuO nanoparticles are synthesized by a sono-chemical synthesis by the assistance of ultrasound with the reaction time up to 30 min and calcination at 600-700 °C [12]. It was additionally revealed that its crystallization and particle size was strongly dependent on the reaction time and calcination temperature (Figure 8).
Ghane M, et al. examined that the novel dry synthetic methods such as thermal decomposition of oxalate precursor for the synthesis of binary nano metal oxides (Copper Oxide) [13]. The synthesis route involves facile solid-phase mechano-chemical activation of a physical mixture of simple copper salts and oxalic acid, followed by calcination of the as-ground oxalate precursors at 450 °C. SEM and XRD data lead to the particle diameters: < 10 nm for CuO (Figure 9).
Ethiraj AS, et al. proposed that by using a simple and inexpensive wet chemical method, copper oxide nanowires prepared with diameters of 90 nm and lengths of several micrometers [14]. The CuO nanowires prepared via this method have wide applications for industrial applications which require mass production and low thermal budget technique. The Fourier transform infrared spectrum analysis, energy dispersive X-ray analysis, X-ray diffraction analysis, and X-ray photoemission spectrum analysis confirm clearly the formation of a pure phase high-quality CuO with monoclinic crystal structure (Figure 10).
Mousa MK proposed fast, inexpensive and simple quick precipitation method and control the size with different temperature; 65,75 and 85 °C. By increasing temperature leads to decrease of nanoparticles size [15]. The sizes of CuO-TOAB stabilized nanoparticles are smaller than CuO non stabilized nanoparticles that prepared at the same temperature. X-Ray diffract to gram peaks showed that all obtained CuO NPs have monoclinic structure. Studying the effect of different concentrations of NaOH reducing agent and TOAB surfactant on the CuO nanoparticles size and morphology and antibacterial activity of CuO nanoparticles with other surfactants can be considered as future work (Figure 11).
Nasibulin AG, et al. [16] studied the thermal decomposition of copper acetylacetonate vapor using a vertical flow reactor at ambient nitrogen pressure for the synthesis of Crystalline nanometersizedcopper (I) oxide particle. They performed experiments in the precursor vapor pressure range of Pprec= 0.06 to 44 Pa at furnace temperatures of 431.5 °C, 596.0 °C, and 705.0 °C. Crystalline product of the decomposition depended on the precursor vapor pressure. The formation of copper (I) oxide particles occurs due to the surface reaction of the decomposition products (mainly carbon dioxide). They proposed a model to build a semi empirical phase diagram of the precursor decomposition products (Figure 12).
Zhu H, et al. proposed a wet chemical method to synthesize stable CuO nanofluids in a large-scale [17]. Different copper salts resulted in different particle morphologies. Size and shape of clusters of primary nanoparticles are affected by concentration of copper acetate and reaction time. By changing the synthesis parameters nanofluids with different microstructures could be obtained. The thermal conductivity of CuO nanofluids increased with the increase of particle loading(Figure 13).
Kannaki K, et al. proposed the hydrothermal route for synthesis of copper oxide nano particles [18]. It is confirmed by XRD analysis. The UV-Visible study shows the radiation in red region can be applicable for fabrication of optoelectronic devices. The FTIR analysis reveals the characterization peaks for CuO stretching. The adsorption of ammonia is confirmed by FTIR spectra. The spherical Morphology is confirmed by SEM/EDS (Figure 14). This method may be suitable for large scale production of CuO nanoparticles for practical applications.
Darezereshki E, et al. spherical CuO nanoparticles with mean diameter of 170±5 nm have been prepared by a thermal decomposition method [19]. This method does not require organic solvents, expensive raw materials and complicated equipment. So, it is concluded that the presented method is superior to the other methods for the synthesis of CuO nanoparticles from dilute CuSO4 solution (Figure 15).
Srivastava S, et al. Cupric oxide (CuO) nanoparticles were prepared by the chemical route by calcinations at a higher temperature from 300 °C to 400 °C [20]. For the comparison transmission electron microscopy (TEM) and x-ray diffraction (XRD) measurements were made through JCPDS. There is good agreement between data produced by spectroscopy and the microscopic measurements.
Ayask HK, et al. synthesize of copper oxide nanoparticles using copper hydroxide by mechanochemical process [21]. The main advantage of this study is CuO nanoparticles with narrow size distribution without subsequent annealing during the process. The results of X-ray diffraction (XRD) indicated that the dehydration of Cu(OH)2 into CuO was completed after three hours of milling. Structural analysis using scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS), transmission electron microscopy (TEM) and particle size analyzer (PSA) showed that CuO particles had moderately equiaxed shape with sizes ranging from 10-27 nm.
According to DTA and DDTA curves, an evident endothermic peak was observed at approximately 180-200 °C. This temperature range corresponds to the dehydration of Cu(OH)2 and the formation of CuO (Figure 16).
Nithya K, et al. Copper oxide nanoparticles were prepared by modified sol-gel technique using sodium dodecyl sulphate as a surfactant [22]. Effect of calcination temperature on particle size, band-gap, crystallinity and morphology of the nanoparticles were studied with the help of particle size analysis, UV-Spectroscopy, powder X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) studies (Figure 17). Prepared nanoparticles will be tested for their activity towards gas sensing.
Karthik AD, et al. the present investigation reports, the novel synthesis of Copper and Copper oxide nanoparticles using Chemical reduction method and its physicochemical characterization [23]. The nanoparticles have been prepared using Copper (II) succinate as precursor. Copper nanoparticles are initially formed and subsequently oxidized to copper oxide. As reported the nanoparticles were characterized by UV-visible spectroscopy, Fourier transform infra-red spectroscopy (FTIR), X-ray diffraction measurements (XRD) and scanning electron microscopy (SEM). SEM analysis exhibited nanoparticles with an average diameter of about 45 nm (Figure 18). XRD analysis revealed broad pattern of fcc crystal structure of copper metal and cubic cuprites structure for Cu2O. The antimicrobial properties of copper nanoparticles were investigated using Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. The bactericidal effects of copper nanoparticles were studied based on diameter of inhibition zone in disk diffusion tests of nanoparticles dispersed in batch cultures. The copper nanoparticles showed excellent activity against Escherichia coli and Staphylococcus aureus, with excellent inhibition zones of 14 mm and 10 mm, respectively. Bacterial sensitivity to nanoparticles was found to vary depending on the microbial species.
Shah MA, et al. temperature and pressure, both of which can affect the super saturation and nucleation are responsible for solvents properties [24]. In this study, we use water as solvent under supercritical conditions and report copper (Cu) and (Cu2O) nanoparticles of size ranging from 9 nm to 60 nm. This synthetic technique has the following advantages: Firstly, it is one step synthesis approach, making it easy to control the growth kinetics. Secondly, the synthesis needs no sophisticated equipment. Thirdly, the approach is non-toxic without producing hazardous waste as water is being used as solvent as well as source of oxygen. Forth, it is a surfactant free synthesis and has bright prospects.
Jayalakshmi, et al. Water-soluble cupric oxide nanoparticles are stable over a wide range of pH and temperature. This excellent stability in the form of aqueous colloidal suspensions makes the application of the water-soluble CuO nanoparticles easier in aqueous systems [25]. In the present study, copper oxide nanoparticles are synthesized using flower extract (aqueous) of Cassia alata L., member belonging to family Fabaceae profusely growing in the wild (Figure 19). The synthesized nanoparticles are subjected to SEM, EDX, XRD to understand the particle characteristics of the copper oxide nanoparticles which closely matched with JCPDC standard (Figure 20).
Rani R, et al. Purewal Monoclinic CuO nanoparticles were synthesized by reverse micelle technique [26]. Size and morphology were characterized by using XRD, and TEM techniques. FTIR technique was used to ensure the bonding between metal and oxygen. Antibacterial activity of CuO nanoparticles were tested against bacterial strains K. pneumoniae, S. typhimurium, and E. aerogenes using the agar diffusion method. Minimum inhibitory concentration of these three gram-negative bacterial strains K. pneumoniae, S. typhimurium, and E. aero genes were found to be 0.55, 0.15, and 0.30 μg/ml, respectively (Figure 21).
Radhakrishnan AA, et al. nanostructured materials have wide range of applications due to their interesting size-dependent chemical and physical properties compared to particles of size in the range of micrometer [27]. Copper oxide nano materials are of interest on account of their potential uses in many technological fields. In this study CuO nanoparticles were synthesized via simple sol gel method using basic CuSO4 as wet chemically synthesized precursor and NaOH as stabilizing agent. Samples were characterized by X-ray diffraction (XRD), infrared spectrum (IR), scanning electron microscope (SEM), transmission electron microscopy (TEM) and UV-visible spectrum (Figures 22 and 23). Using this method CuO nanoparticle could be synthesized without using organic solvent, expensive raw materials and complicated equipments. Besides simplicity, the advantage of producing nanoparticles by this method is that it is easeful, flexible, fast, cost effective, and pollution free.
Devi HS, et al. this work is to report an environmental benign route for the fabrication of copper oxide nanoparticles using Centella asiatica (L.) leaves extracts at room temperature [28]. This method is completely a green method, free from toxic and harmful solvent. Copper oxide particles such prepared are in nanoscale and there morphology and size are characterized using SEM, UV-Visible spectroscopy, IR spectroscopy and EDX. Copper oxide nanoparticles synthesized by this method can be used for the photo catalytic degradation of methyl orange. These nanoparticles can reduce methyl orange to its leuco form in aqueous medium in the absent of reducing agents. It is more economy as compare to other methods. This catalytic effect of copper oxide nanoparticles can be contributed to its small size. Nanoparticles have many active sites as compared to the bulk materials because of its large surface to volume ratio. Copper oxide nanoparticles such prepared has good catalytic properties.
Ahamed M, et al. it describes the structural and antimicrobial properties of copper oxide nanoparticles (CuO nanoparticles) synthesized by a very simple precipitation technique [29]. Copper (II) acetate was used as a precursor and sodium hydroxide as a reducing agent. X-ray diffraction patter (XRD) pattern showed the crystalline nature of CuO nanoparticles. Field emission scanning electron microscope (FESEM) and field emission transmission electron microscope (FETEM) demonstrated the morphology of CuO nanoparticles. The average diameter of CuO nanoparticles calculated by TEM and XRD was around 23 nm. Energy dispersive X-ray spectroscopy (EDS) spectrum and XRD pattern suggested that prepared CuO nanoparticles were highly pure (Figure 24). CuO nanoparticles showed excellent antimicrobial activity against various bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterococcus faecalis, Shigella flexneri, Salmonella typhimurium, Proteus vulgaris, and Staphylococcus aureus). Moreover, E. coli and E. faecalis exhibited the highest sensitivity to CuO nanoparticles while K. pneumonia was the least sensitive. Possible mechanisms of antimicrobial activity of CuO nanoparticles should be further investigated.
Shaffiey SF, et al. CuO is one of the most important transition metal oxides due to its captivating properties [30]. It is used in various technological applications such as high critical temperature superconductors, gas sensors, in photoconductive applications, and so on. Recently, it has been used as an antimicrobial agent against various bacterial species. Materials and methods: Here, we synthesized CuO nanoparticles and explored the antibacterial activity of CuO preparation. Results: Single crystalline nanoparticles of copper oxide having almost uniform particle size of 5-6 nm has been synthesized by a facile and versatile route. XRD spectra confirmed the formation of single phase CuO nanoparticles. Transmission electron microscopy results corroborate well with XRD results. The technique employed is free from toxic solvents, organics and amines, is based on a simple reaction of copper sulfate and de-ionized water (DI), and their bactericidal effects against of Aeromonas hydrophila ATCC 7966T bacteria were investigated. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) with liquid culture for all of the Aeromonas hydrophila culture medias was done. Conclusion: Present study confirms that Copper oxide nanoparticles have great promise as antimicrobial agent against Aeromonas hydrophila.
Gondal MA, et al. pulsed laser ablation in liquids is a simple synthesis process of nanoparticles for the production of high purity material with no need for any expensive instrumentation except laser [31]. The 532 nm wavelength laser beam with 5 ns pulse width and 10 Hz repetition rate was an ablating laser source. In order to control the size and stoichiometry of the nano particles, the laser ablation was done in the presence of 9% of H2O2. The optical properties and structure of the prepared samples were studied using different analytical techniques, such as energy dispersive X-ray spectroscope (EDS), X-ray Diffraction, UV-Visible absorption, Photoluminescence, FT-IR. In order to study the morphology of the prepared nano-sized powders, Field Emission Scanning Electron Microscope was used. From the above analytical studies it was found that the particle size was between 13 and 28 nanometer, while the band gap energy was estimated to be 2.46 eV (Figure 25).
Wua R, et al. In this paper, highly dispersed copper oxide (CuO) nanoparticles with different size and morphology have been successfully prepared by a quick-precipitation method in DMACwater mixed solvent [32]. The as-prepared CuO nanoparticles were characterized by high resolution transmission electron microscopy (HRTEM) and x-ray diffraction (XRD) (Figure 26). Several kinds of CuO particles with different morphology were obtained including panicle-shaped, spherical-shaped, spindly-shaped and rod-shaped CuO particles. The effects of processing parameters on the size and shape of CuO particles such as volume ratio of DMAC/water, adding temperature of NaOH and molar ratio of were investigated. The nucleation and growth kinetic of the resulting CuO particles were also discussed.
Deng D, et al. copper nanoparticles are alternative for silver and gold nanoparticles which are currently used in inkjet printing of conductive patterns because of the low price and high electrical conductivity [33]. However, the serious impediment to using copper nanoparticles for conductive inks is their spontaneous oxidation. In the paper, the well-dispersed anti oxidative copper pastes were prepared by dispersing the nanoparticles in an ethanol solution of lactic acid, and then deposited on glass slides. The resistivity of conductive copper films was lAx10-5 n·cm after annealing at 200 °C for 30 min. It was experimentally proved that lactic acid could react with the copper oxides surrounding Cu to form copper carboxylate, which was then reduced to Cu after annealing at 200 °C under nitrogen atmosphere. Furthermore, the copper film after annealing at 200 °C for 30 min under nitrogen atmosphere showed anti oxidative characteristic (Figure 27).
Waichal RP, et al. Cuprous oxide (Cu2O) nanoparticles with size of about 32 nm are synthesized via electrochemical method in NaCl solution with copper electrodes and K2Cr2O7 as an additive [34]. Different techniques such as scanning electron microscopy (SEM), energy dispersive x-ray analysis (EDAX), x-ray Diffraction (XRD), transmission electron microscopy (TEM) and electron spectroscopy for chemical analysis (ESCA) are used for sample characterization. A universal miniature furnace is designed and developed for gas sensing application. It is made with alumina (Al2O3) tube with 0.5 mm inner diameter, 1 mm outer diameter and 1 cm length and has capacity to reach up to 3500 C. Calibration of bed/furnace is done with Keithley 2400 C source meter with Lab tracer 2.0 software. Cu2O nanoparticle coating (thickness around 10 microns) is formed on the surface of the miniature alumina furnace, with thin platinum wire used as electrode. The current Vs. % RH of P type Cu2O exhibits linear change in current from 3.83 X 10-7 to 2.62 X10-7 Amps for a relative humidity change from 11 to 84% RH, respectively.
Park JC, et al. in this paper, synthesis of uniform Cu2O nanocubes in a gram scale [35]. The controlled oxidation of Cu2O nanocubes yielded CuO hollow cubes, hollow spheres, and urchin-like particles, through a sequential dissolution-precipitation process. The CuO urchin-like particles exhibited excellent electrochemical performance for lithium-ion batteries, superior to that of the other nanostructures. It is anticipated that precise control of the morphology of metal oxide nanoparticles would serve to maximize the performance of lithiumion batteries. Experiments with different metal oxides, such as FeO, CoO, and Co3O4, are in progress toward this goal (Figure 28).
Rout L, et al. this paper describes the CS cross coupling of thiols with iodobenzene in the presence of relatively inexpensive air stable CuO nanoparticles [36]. A variety of thiols could be cross-coupled with iodobenzene in this simple and efficient reaction to give the desired products in high yields.
Etefagh R, et al. copper oxide (CuO) nanoparticles and nanolayers were synthesized by sol-gel and spray pyrolysis methods, respectively [37]. The structure and morphology of the prepared samples were characterized using XRD, SEM and TEM analysis. Aspergillus niger fungi were grown in an appropriate medium and exposed to the synthesized samples in a closed glass vessel. The boisoning properties of the nanosystems were investigated by measuring their electrical resistance at regular time intervals and different temperatures. Further studies were made on the effects of CO2 and humidity on the sensing properties, using CaCO3 and Silica gel. The considerable changes observed in the electrical resistance of the prepared samples, in the presence of Aspergillus niger fungi, support our proposed system as a biosensor.
Phiwdanga K, et al. CuO nanoparticles were synthesized by precipitation method using different precursors as copper nitrate (Cu(NO3)2) and copper chloride (CuCl2) with post-heating comparing between as-synthesized and after calcinations [38]. Relevant properties of as-synthesized nanoparticles were investigated by X-ray diffraction (XRD), scanning electron microscope (SEM) and Fourier Transform Infrared (FTIR) Spectroscopy (Figure 29). Overall results suggest that the formation of CuO nanostructures with different shape, size and morphology can be achieved using different precursors via this process. The improvement in their crystallinity and purification can be further attained by post calcinations process.
Lo1 CH, et al. the optimal parameters are found for preparing nanofluid in our submerged arc nanoparticle synthesis system (SANSS) using a copper electrode [40]. A suspended copper oxide nanofluid is thus produced at the current of 8.5-10 A, voltage of 220 V, pulse duration of 12l s, and dielectric liquid temperature of 2 °C. The CuO nanoparticle are characterized by transmission electron microscopy (TEM), field emission scanning electron microscope (FESEM), X-ray diffraction (XRD), electron diffraction pattern (SAD) and electron spectroscopy for chemical analysis (ESCA). The equality volume spherical diameter of the obtained copper oxide particle is 49.1 nm, regular shape and narrow size distribution (Figure 30).
Yao WT, et al. uniform and monodisperse CuO nanorods have been synthesized by directional aggregation and crystallization of tiny CuO nanoparticles generated from a solid-liquid arc discharge process under ambient conditions in the absence of any surfactants [40]. Uniform CuO nanorods with sharp ends are formed from tiny nanoparticles via a process that involves the rapid oxidation of Cu nanoclusters, the spontaneous aggregation of CuO nanoparticles, and the Ostwald ripening process. The spontaneous aggregation and oriented attachment of tiny CuO nanoparticles contributed obviously to the formation of these kinds of nanostructures. By choice of suitable reducing agent to prevent the oxidation of Cu nanoclusters, Cu and Cu2O nanoparticles can be selectively synthesized (Figure 31).
Conclusion
The key applications of copper oxide nanoparticles are as follows:• As burning rate catalyst in rocket propellant. It can greatlyimprove the homogeneous propellant burning rate, lower pressure index, and also perform better as a catalyst for the AP composite propellant.
• Can be applied to the catalyst, superconducting materials, thermoelectric materials, sensing materials, glass, ceramics and other fields.
• As ceramic resistors, magnetic storage media, gas sensors, near-infrared tilters, photoconductive and photo thermal applications.
• As semiconductors, solar energy transformation, and hightech superconductors.
References
- Guajardo-Pachecoa MJ, Morales-Sanchz JE, González-Hernándezc J, Ruiz F (2010) Synthesis of copper nanoparticles using soybeans as a chelant agent. Mater Lett 64: 1361-1364.
- Xi Y, Hu C, Gao P, Yang R, He X, et al. (2010) Morphology and phase selective synthesis of CuxO (x = 1, 2) nanostructures and their catalytic degradation activity. Mater Sci Eng B 166: 113-117.
- He Y (2007) A novel solid-stabilized emulsion approach to CuO nanostructures microspheres. Mater Res Bull 42: 190-195.
- Motogoshi R, Oku T, Suzuki A, Kikuchi K, Kikuchi S, et al. (2010) Fabrication and characterization of cupprious oxide: fullerene solar cells. Synth Met 160: 1219-1222.
- Mustafa G, Tahir H, Sultan M, Akhtar N (2013) Synthesis and characterization of cupric oxide (CuO) nanoparticles and their application for the removal of dyes12: 6650-6660.
- Suleiman M, Mousa M, Hussein A, Hammouti B, Hadda TB, et al. (2013) Copper(II)-Oxide nanostructures: synthesis, characterizations and their applications-review. J Mater Environ Sci 4: 792-797.
- Aparna Y, Rao KV, Subbarao PS (2012) Synthesis and characterization of cuo nano particles by novel sol-gel method. IPCBEE 48: 156-160.
- Abboud Y, Saffaj T, Chagraoui A, Bouari AE, Brouzi K, et al. (2014) Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl Nanosci 4: 571-576.
- Lanje AS, Sharma SJ, Pode RB, Ningthoujam RS (2010) Synthesis and optical characterization of copper oxide nanoparticles. Adv Appl Sci Res 1: 36-40.
- Volanti DP, Keyson D, Cavalcante LS, Simões AZ, Joya MR, et al. (2008) Synthesis and characterization of CuO flower-nanostructure processing by a domestic hydrothermal microwave. J Alloys Comp 459: 537-542.
- Sahay R, Sundaramurthy J, Kumar PS, Thavasi V, Mhaisalkar SG, et al. (2012) Synthesis and characterization of CuO nanofibers, and investigation for its suitability as blocking layer in ZnO NPs based dye sensitized solar cell and as photocatalyst in organic dye degradation. J Solid State Chem 186: 261-267.
- Wongpisutpaisan N, Charoonsuk P, Vittayakorn N, Pecharapa W (2011) Sonochemical synthesis and characterization of copper oxide nanoparticles. Energy Procedia 9: 404-409.
- Ghane M, Sadeghi B, Jafari AR, Pakenjhad AR (2010) Synthesis and characterization of a Bi-Oxide nanoparticle ZnO/CuO by thermal decomposition of oxalate precursor method. Int J Nano Dim 1: 33-40.
- Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7: 70.
- Mousa MK (2015) Wastewater disinfection by synthesized copper oxide nanoparticles stabilized with surfactant. J Mater Environ Sci 6: 1924-1937.
- Nasibulin AG, Ahonen PP, Richard O, Kauppinen EI, Altman IS (2001) Copper and copper oxide nanoparticle formation by chemical vapor nucleation from copper (II) acetylacetonate. J Nanopart Res 3: 383-498.
- Zhu H, Han D, Meng Z, Wu D, Zhang C (2011) Preparation and thermal conductivity of CuO nanofluid via a wet chemical method. Nanoscale Res Lett 6: 181.
- Kannaki K, Ramesh PS, Geetha D (2012) Hydrothermal synthesis of CuO nanostructure and their characterizations. Int J Sci Eng Res 3: 1-4.
- Darezereshki E, Bakhtiari F (2011) A novel technique to synthesis of tenorite (CuO) nanoparticles from low concentration CuSO4 solution. J Min Metall B Metall 47: 73-78.
- Srivastava S, Kumar M, Agrawal A, Dwivedi SK (2013) Synthesis and characterisation of copper oxide nanoparticles. IOSR J Appl Phys 5: 61-65.
- Ayask HK, Khaki JV, Sabzevar MH (2015) Facile synthesis of copper oxide nanoparticles using copper hydroxide by mechanochemical process. J Ultrafine Grained Nanostruct Mater 48: 37-44.
- Nithya K, Yuvasree P, Neelakandeswari N, Rajasekaran N, Uthayarani K, et al. (2014) Preparation and characterization of copper oxide nanoparticles. Int J Chem Tech Res 6: 2220-2222.
- Karthik AD, Kannappan G (2013) Synthesis of copper precursor, copper and its oxide nanoparticles by green chemical reduction method and its antimicrobial activity. J Appl Pharm Sci 3: 016-021.
- Shah MA, Al-Ghamdi MS (2011) Preparation of copper (Cu) and copper oxide (Cu2O) nanoparticles under supercritical conditions. Mater Sci Appl 2: 977-980.
- Jayalakshmi, Yogamoorthi A (2014) Green synthesis of copper oxide nanoparticles using aqueous extract of flowers of Cassia alata and particles characterisation. Int J Nanomater Bios 4: 66-71.
- Rani R, Kumar H, Salar RK, Purewal SS (2014) Antibacterial activity of copper oxide nanoparticles against gram-negative bacterial strain synthesized by reverse micelle technique. Int J Pharm Res Dev 6: 72 -78.
- Radhakrishnan AA, Beena BB (2014) Structural and optical absorption analysis of CuO nanoparticles. Indian J Adv Chem Sci 2: 158-161.
- Devi HS, Singh TD (2014) Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv Electron Electric Eng 4: 83-88.
- Ahamed M, Alhadlaq HA, Khan MA, Karuppiah P, Al-Dhabi NA (2014) Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J Nanomater 2014: 4.
- Shaffiey SF, Shapoori M, Bozorgnia A, Ahmadi M (2013) Synthesis and evaluation of bactericidal properties of CuO nanoparticles against Aeromonas hydrophila. Nanomed J 1: 198-204.
- Gondal MA, Qahtan TF, Dastageer MA, Saleh TA, Maganda YW (2013) Synthesis and characterization of copper oxides nanoparticles via pulsed laser ablation in liquid. High Capacity Optical Networks and Emerging/ Enabling Technologies, Magosa, pp. 146-150.
- Wua R, Zhenye Ma, Zhenggui Gua, Yan Yang (2010) Preparation and characterization of CuO nanoparticles with different size and morphology. Mechanic Automation and Control Engineering (MACE), 2010 International Conference on, Wuhan, pp. 3183-3186.
- Deng D, Qi T, Chen Y, Jin Y, Xiao F (2012) Preparation of antioxidative nano copper pastes for printed electronics application. Electronic Packaging Technology and High Density Packaging (ICEPT-HDP), 2012 13th International Conference on, Guilin, pp. 250-253.
- Waichal RP, Karvir GD, Patil KR, Ambekar P, Mulla IS, et al. (2012) Synthesis of cuprous oxide nanoparticles by electrochemical method and evaluation of the corresponding nanoparticle film for humidity sensing. Physics and Technology of Sensors (ISPTS), 2012 1st International Symposium on, Pune, India, pp. 47-50.
- Park JC, Kim J, Kwon H, Song H (2009) Gram-scale synthesis of Cu2O nanocubes and subsequent oxidation to CuO hollow nanostructures for lithium-ion battery anode materials. Adv Mater 21: 803-807.
- Rout L, Sen TK, Punniyamurthy T, (2007) Recent advances in coppercatalyzed C-S cross-coupling reactions. Angew Chem 46: 5583-5586.
- Etefagh R, Azhir E, Shahtahmasebi N (20113) Synthesis of CuO nanoparticles and fabrication of nanostructural layer biosensors for detecting Aspergillus niger fungi. Scientia Iranica 20: 1055-1058.
- Phiwdang K, Suphankij S, Mekprasart W, Pecharapa W (2013) Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 34: 740-745.
- Lo CH, Tsung TT, Chen LC, Su CH, Lin HM (2005) Fabrication of copper oxide nanofluid using submerged arc nanoparticle synthesis system (SANSS). J Nanopart Res 7: 313-320.
- Yao WT, Yu SH, Zhou Y, Jiang J, Wu QS, et al. (2005) Formation of uniform CuO nanorods by spontaneous aggregation: selective synthesis of CuO, Cu2O, and Cu nanoparticles by a solid-liquid phase arc discharge process. J Phys Chem B 109: 14011-14016.