Journal of Analytical & Molecular Techniques
Download PDF
Research Article
Parboiled Preservation of Odonata Nymphs for DNA Related Research
Sutherland LN1*, Fomekong-Lontchi J1,5*, Lupiyaningdyah P1,4, Tennessen KJ3, Carter P1 and Bybee SM1,2
1Department of Biology, Brigham Young University, 4102 LSB,
Provo, UT 84602, USA
2Bean Life Science Museum, Brigham Young University, Provo, UT 84602, USA
3Florida State Collection of Arthropods, Gainesville, Florida, USA
4Research Center for Biosystematics and Evolution, National Research and Innovation Agency (BRIN), Cibinong, West Java, Indonesia
5Institute of Medical Research and Medicinal Plants Studies (IMPM), Centre of Medical Research, P.O Box: 6163 Yaoundé, Cameroon
2Bean Life Science Museum, Brigham Young University, Provo, UT 84602, USA
3Florida State Collection of Arthropods, Gainesville, Florida, USA
4Research Center for Biosystematics and Evolution, National Research and Innovation Agency (BRIN), Cibinong, West Java, Indonesia
5Institute of Medical Research and Medicinal Plants Studies (IMPM), Centre of Medical Research, P.O Box: 6163 Yaoundé, Cameroon
*Address for Correspondence:Sutherland LN, Department of Biology, Brigham Young University,
4102 LSB, Provo, UT 84602, USA E-mail Id: lns25@byu.edu
Submission:09 December, 2024
Accepted:15 January, 2025
Published:20 January, 2025
Copyright: ©2025 Sutherland LN, et al. This is an open access
article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Keywords:Dragonfly; Damselfly; Nymphs; DNA; Parboil
Abstract
Odonate nymph preservation is less standardized and more prone to
long-term preservation issues than adults.Two preemptive measures that
can be taken to help prevent the high level of decay often seen in older
nymph specimens are 1. Ethanol injection 2. Parboiling. Parboiling offers
the long-term advantage of better retaining the morphology, which is a great
advantage for future taxonomic projects. Here we found that parboiling is as
good for preserving DNA as ethanol injection.
Introduction
Natural history collections have been the backbone of systematic
and taxonomic research by housing well-preserved specimens that
offer repeatability and comparability in phenotypic analyses [1]. The
importance of natural history collections has received a growing
amount of attention [1-6] in the face of the biodiversity crisis and
continual underfunding. This resurgence in museum interest is
due in part to the increase in digitization efforts and advances in
technology that allows for the sharing of data at a global scale [7].
Museum specimens have long been used in large-scale evolutionary,
biodiversity, and ecological research, but the increase in publicly
available data has created opportunities for broader and more
collaborative research to take place [8]. Recently, there have been calls
to address issues (e.g., lack of administrative support, understaffing,
declines in specimens being deposited, specimen degradation) in
natural history collections to set them up for success in this next
phase of broader museum-based research [4,1]. One way to ensure
museum-based research will continue long into the future is to
consistently use best practices for long-term preservation and storage
methods, which will vary by group.
In general, insect collections are extensive, and odonates
(dragonflies and damselflies) collections are no exception. For
instance, a recent survey was conducted at 13 institutions that possess
at least 100 odonate-type specimens [9]. Among these are the Florida
State Collection of Arthropods (~1.1 million specimens), the Naturalis
Biodiversity Center (~200,000 specimens), and the Natural History
Museum at London (~110,000 specimens) demonstrating the sheer
size of odonate collections. Due to the scale of these collections and
limited expertise, there is often a backlog of processing and taxonomic
work where specimens potentially sit for years, so morphological and
genetic preservation is critical.
Odonata is a medium-sized hemimetabolous order that has
become highly studied and collected due to brilliant coloration,
charismatic behavior, and aquatic lifestyle. Historically, odonates
have been used as biological indicators of freshwater health [10-13]
and often collected as nymphs in large biodiversity projects and
deposited in museums for later identification. While adult taxonomy
is relatively well known, nymph taxonomy is greatly understudied,
with less than half of the recognized species having a documented
nymph, as more effort has traditionally been spent on rearing nymphs
to adults for identification [14]. While nymph taxonomy is greatly
lagging behind adult taxonomy, there has been an effort to close this
gap in recent decades. For example, the majority of species in Ischnura
are known compared to approximately 20% of Telebasis. Historically,
more work has been done on Anisoptera than Zygoptera, but early
nymph descriptions are often brief, lacking detailed illustrations, and
in need of reexamination, highlighting the need for well-preserved
nymph specimens in collections.
Adult odonates preservation has become standardized with
three methods. First, specimens are air-dried and stored in glassine
envelopes without any additional preservation measures, which
allows for the coloration to fade over time and provides inconsistent
results in genetic studies. Second, the specimens are adjusted (i.e.,
wings folded above the abdomen and legs stretched) and then soaked
in acetone for 12-14 hours after which they are dried and again stored
in glassine envelopes. Acetone is excellent for preserving coloration
which is an important character in adult odonate taxonomy and
evolutionary research. Lastly, adults are stored in 70%-95% ethanol.
Ethanol preservation is ideal for genetic studies but can cause
significant color fading if left in the light.
Nymph preservation is less standardized and more prone to longterm
preservation issues than adults. Nymphs generally are preserved
straight into 70-95% ethanol, but ethanol does not readily penetrate
the cuticle, and often results in the specimen decaying to varying
degrees [15,16]. Perforations are often made in the abdomen of large
specimens in an attempt to help the ethanol penetrate the specimen,
but this compromises morphological features that could be needed for
identification in the future. Also, neglecting to refresh the ethanol will
affect the long-term preservation of DNA. There are two preemptive
measures (ethanol injection and parboiling) that can be taken to help
prevent this high level of decay. Ethanol injection, a fairly common
preemptive measure, is when 95% ethanol is directly injected into the
specimens upon capture. Parboiling, a technique not often utilized in
odonates, consists of dispatching the specimen by placing it briefly in
boiling water [14]. Boiling insects fixes the proteins in the body and
prevents them from decaying over time [17].
Parboiling offers the long-term advantage of retaining the
morphological dimensions and patterns of the specimens, closer to
what they are under living conditions, which is a great advantage
for future taxonomic projects. The benefits of parboiling have been
demonstrated in other groups. For example, caterpillars, if not boiled,
will decompose and turn black [18,19]. Parboiling has additionally
been shown to be effective in forensic research when estimating postmortem
intervals as it keeps the size, coloration, and internal organs
of larvae closer to their natural states [20]. Although parboiling
provides long-term stability of morphological features in odonate
nymphs (KTJ, personal comment) it is still unclear how efficiently it
preserves genetic material. Here we aim to
1. Test if there is a difference between the DNA quantity
captured based on the initial preservation method (parboiled
vs. ethanol injected) in odonate nymphs and
2. Test if there is a difference in DNA degradation (fragment
length) between the initial preservation methods.
Materials and Methods
Specimen preservation and sampling:
Odonate nymphs were collected from multiple locations in
Wisconsin, and one location in New York. Freshly collected nymphs
were initially preserved in one of two ways: ethanol injection or
parboiling. Parboiling consisted of submerging the nymph in boiling
water for 30–60 seconds, depending on size. All specimens, regardless
of initial preservation method, were stored in 80% ethanol in a -20°Cfreezer. Thirty-eight specimens were selected for extraction which
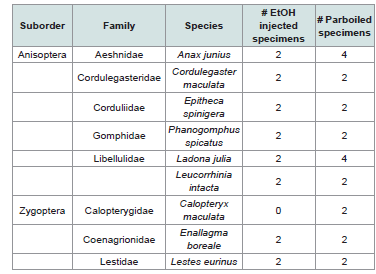
represent nine species (6 Anisoptera, 3 Zygoptera) [Table 1].
DNA Extraction and Quantification:
For each species at least four specimens (two per initial
preservation method) were extracted, except for Calopteryx maculata
where no ethanol injected specimen was available [Table 1]. Each
specimen was extracted three times (pro-, meso- and metathoracic
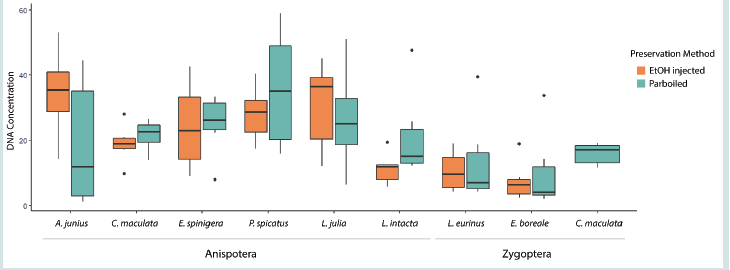
legs) and the variation in concentration between species is depicted
(Figure 1). DNA extractions were performed using a Qiagen DNeasy
Blood & Tissue kit (Valenica, CA) following manufacturer protocols,
with two exceptions. The sample was incubated at 56°C for 72 hours
during tissue lysis and the final elution volume was changed to 25 μL
which resulted in a final volume of ~45 μL. A Qubit 4 fluorometer
was used to quantify the DNA concentration with the dsDNA High
sensitivity procedure.DNA Degradation:
To test for DNA degradation over time, specimens were reextracted
in 2024, three years after the initial extractions. In total
32 extractions were selected, 16 extractions (8 ethanol injected, 8
parboiled) from 2021 and 16 new extractions performed in 2024.
All but Lestes eurinus was re-extracted. As an estimate for DNA
degradation, a fragment analysis was performed using the Agilent
Genomic 55 kb BAC Kit Quick Guide for Femto Pulse Systems at the
BYU DNA Sequencing Center. Two parboiled extractions from 2024
(Calopteryx maculata and Leucorrhinia intacta) were removed from
further analysis as no fragment length was recovered.Statistical analysis:
To test for a difference in DNA concentration between the initial
preservation methods (ethanol injected and parboiled), a Mann-
Whitney U Test was run in Rstudio v. 4.4.1 using the wilcox.test
function in the dplyr package [21]. To test the stability of the genetic
material by initial preservation two Mann-Whitney U Tests were run,
one for each year (2021 and 2024).Results
While there is variation in the DNA concentration between
species [Figure 1], in general, the average DNA concentration (ng/μl)
was higher for species in Anisoptera (24.3) than Zygoptera (11.5). The
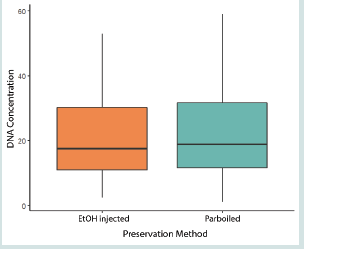
average DNA concentration was 20.7 and 21.0 for ethanol injected
and parboiled specimens, respectively. The results of the Mann-
Whitney U Test showed that there is no significant difference (W
= 1590.5, p-value = 0.97) in DNA concentration recovered between
parboiled and ethanol-injected specimens [Figure 2].
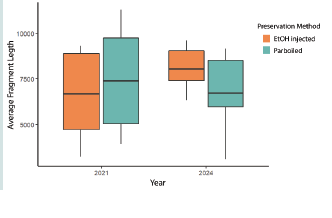
For the extractions completed in 2021, the average fragment
length (bp) for ethanol injected specimens was 6,620 compared to
7,529 for the parboiled specimens. However, based on the results of
the Mann-Whitney U Test, there is no difference (W = 25, p-value =
0.49) in fragment length between the methods. For the extractions
completed in 2024, the average fragment length (bp) for ethanol
injected specimens was 8,113 compared to 6,754 for the parboiled
specimens. The results of the Mann-Whitney U Test for the 2024
extractions indicate that there is no difference (W = 33, p-value =
0.27) in fragment length between the methods [Figure 3].
Discussion
The results above demonstrate that there is no difference in DNA
quantity or quality between initial preservation methods (ethanol
injected and parboiling) over time. Therefore, to better preserve
nymph morphology, parboiling should be the standard practice.
Parboiling has the benefit of slowing down degradation caused by
enzymes, which helps preserve the morphological integrity of the
nymph [20]. For long-term storage, nymphs should be kept in either
high concentration ethanol (95-100) or -80°C freezers with a lower
ethanol concentration (70-100). While both have issues, such as
brittle specimens leading to a high probability of breakage for high
concentration ethanol specimens, and DNA degradation due to the
rapid freezing and thawing when working with -80°C freezers, they
are still the current best practices [22,23].
Nymph taxonomy and identification are based almost solely on
the final instar. However, with the high level of adult sequences that
are publicly available, it is possible to associate unknown nymphs
at various stages with relative ease and accuracy. This approach
will allow researchers to get better insight into the different instars,
there by expanding our understanding of nymph development,
morphology, and taxonomy. Additionally, it will allow for increased
use of museum s pecimens, as more nymphs will be utilized which are
not at final instar. The benefits will be increased if parboiled specimens
are utilized, as a more detailed description can be provided at the end.
Relatively little is known about which morphological nymph
characters demonstrate phylogenetic synapomorphies across the
order, although some integrative studies have appeared [24-27].
Nymph characters have been found to help resolve generic limits
in the past [28-30]. Questions remain regarding whether certain
mouthparts (i.e.,premental and mandibular structure), thoracic
morphology, and lateral abdominal and anal gills show relationships
within or between groups [29,14]. Therefore, nymph preservation
is critical to all stages of systematic, phylogenetic, and evolutionary
research.
Acknowledgments
We would like to thank the BYU undergraduates who helped with
DNA extractions and the BYU sequencing center for performing the
fragment analysis. We also thank Dr. Dennis Paulson for information
on the importance of insect collections for many fields of biology.