Journal of Cardiobiology
Download PDF
Research Article
Myristoylated Protein Kinase C Epsilon Inhibitor Preserves Renal Function in a Mouse Model of Acute Bilateral Kidney Ischemia-Reperfusion Injury
Sleeper S1, Shah L4, Verwoert AB1, Singh SG1, Dean TC1, Johnson D1, Kalu U5, Tanoh DB2,MelnikJ1, Chen Q1, Barsotti R1, Jiang Y3, George JF3, Agarwal A3, and Young L1,2*
1Philadelphia College of Osteopathic Medicine, Philadelphia, USA
2YoungTherapeutics, LLC, Philadelphia, USA
3University of Alabama at Birmingham School of Medicine, Birmingham, AL, USA
4Rowan University, Glassboro, NJ, USA
5Northeast Georgia Medical Center, Gainesville, GA, USA
2YoungTherapeutics, LLC, Philadelphia, USA
3University of Alabama at Birmingham School of Medicine, Birmingham, AL, USA
4Rowan University, Glassboro, NJ, USA
5Northeast Georgia Medical Center, Gainesville, GA, USA
*Address for Correspondence:Lindon Young, Philadelphia College of Osteopathic Medicine,
Philadelphia, USA, Email Id: lindonyo@pcom.edu
Submission:17 May, 2024
Accepted:21 June, 2024
Published:24 June, 2024
Copyright: © 2024 Sleeper S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Keywords:Delayed Graft Function; Ischemia Reperfusion; Kidney;
Protein Kinase C Epsilon; YT-001
Abstract
Delayed graft function (DGF) is a post kidney transplant
complication in which kidney function and urine production is delayed
for days, weeks or months. During this period, the patient must be
maintained on dialysis. DGF is a consequence of ischemia reperfusion
(I/R) injury as the kidney is exposed to ischemia when harvested and
reperfusion once transplanted. The re-introduction of oxygenated
blood underlies I/R damage. DGF occurs in up to 30% of kidney
transplant recipients, which suggests that therapeutic approaches are
needed to improve patient outcomes.
This study investigates the role of protein kinase C epsilon (PKCε)
inhibition in maintaining kidney function in a murine model of bilateral
kidney I/R. Measurements of glomerular filtration rate (GFR) and
serum creatinine (Cr) were used to assess kidney function. Ischemia
was induced in male C57BL/6J mice by clamping kidney pedicles
bilaterally for 19 minutes followed by 96 hours of reperfusion. A cell
permeable peptide that inhibits PKCε (N-myristic acid-EAVSLKPT;
YT-001) interaction with downstream receptors,given at the time
of reperfusion, significantly maintained GFR, blunted serum Cr
elevation to a greater extent, and prevented PKCε translocation to
renal epithelial cell membranes compared to the scrambled control
peptide (N-Myr-LSETKPAV).
These findings suggest that YT-001 given upon the re-institution
of blood flow after kidney transplant would potentially decrease the
incidence of DGF and the subsequent downstream morbidity and
mortality. Based upon these findings, YT-001 was granted Orphan
Drug Status by the FDA in 2023.
Abbreviations
3,3’-Diaminobenzidine: DAB; Acute kidney injury: AKI;
Creatinine: Cr; Delayed graft function: DGF; Dihydrobiopterin: BH2;
Endothelial nitric oxide synthase: eNOS; Extracorporeal shockwave
lithotripsy: ESWL;Glomerular filtration rate: GFR;Hydrogen peroxide:
H2O2;Molecular weight: MW;Myristoylated: Myr;Phosphate
buffered saline: PBS; Protein kinase C epsilon: PKCε;Reactive
oxygen species: ROS;Receptor for activated C kinase-1: RACK-1;
Transactivator of transcription: TAT;Tetrahydrobiopterin: BH4
Introduction
Ischemic injury occurs when tissues are subjected to a cessation
of blood flow leading to cellular dysfunction and damage. Ischemic
injury leads to a reduction of ATP from the mitochondria, which
compromises Na+/K+ ATPase and, in turn, leads to acute renal failure
that is indicated by decreased glomerular filtration rate (GFR) and
increased serum creatinine (Cr). Timely restoration of blood flow
(i.e., reperfusion) to the kidney is essential to restore kidney function.
However, reperfusion of blood flow after prolonged ischemia leads
to the generation of reactive oxygen species (ROS) in blood thatwill
exacerbate injury to the previously ischemic tissue, lead to kidney
fibrosis and compromise renal function months later [1,2]. Hence,
Ischemia-reperfusion (I/R) in the kidney is a well-recognized
mechanism of injury following kidney transplant [1]. There are over
twenty thousand kidney transplants per year in the United States
alone, and approximately 30% of transplant recipients experience
acute kidney injury (AKI) which progresses to the clinical diagnosis
of delayed graft function (DGF) within one week after transplantation
[2].
DGF is characterized by endothelial dysfunction, inflammation,
and ROS. These processes culminate in significant kidney damage,
manifesting as inadequate urine production. Current interventions
are largely supportive with fluid and electrolyte management via
dialysis; however, these measures do not address the mechanisms
of I/R injury to prevent or mitigate DGF [3-9]. Patient outcomes
following DGF include the need for dialysis, graft rejection, and high
morbidity compared to those without DGF [10-12].
In previous in vivo porcine myocardial I/R studies, YT-001, given
at the beginning of reperfusion resulted in a marked reduction in
infarct area and restoration of post-reperfusion cardiac function [13].
YT-001 is known to confer protection by inhibiting the generation of
ROS from uncoupled endothelial nitric oxide synthase (eNOS) and
mitochondrial ATP-sensitive potassium channels [14-19]. YT-001
reduced serum ROS (e.g., H2O2) levels when given at reperfusion in
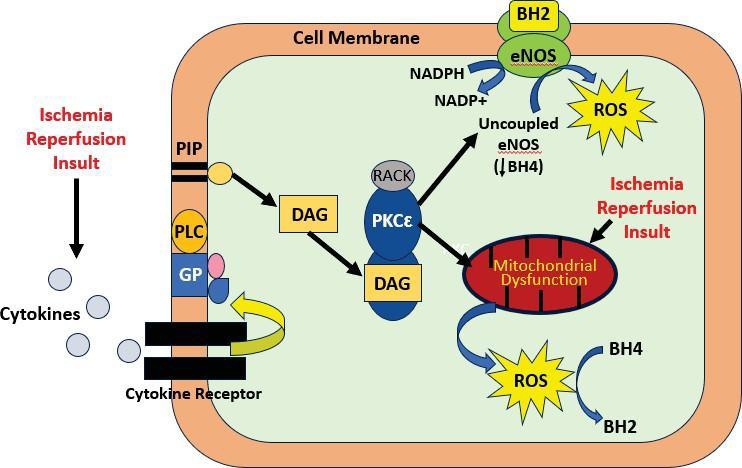
an in vivo rat hindlimb I/R model [14,15]. [Figure 1] illustrates the
pathway following I/R induced ROS injury in the heart and proposed
pathway for the kidney. Extracorporeal shockwave lithotripsy
(ESWL) is a procedure used to break up kidney stones and causes
ischemic-like injury during the 15-minute ESWL procedure. YT-001
attenuated ESWL-induced kidney I/R injury, in part, by preventing
the generation of ROS in blood flow to the kidney [20].
I/R insult activates PKCε via inflammatory cytokines and opening
of mitochondrial ATP-sensitive potassium channels, resulting in
mitochondrial ROS production. Oxidative stress leads to oxidation
of tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2) which
promotes eNOS uncoupling. Increased plasma BH2 levels promote
uncoupled eNOS activity to produce ROS in lieu of nitric oxide when
activated byPKCε phosphorylation [21].
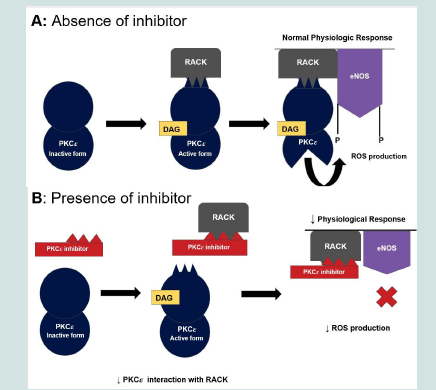
YT-001 prevents the interaction between activated PKCε and its
receptor for activated C kinase-1 (RACK-1) resulting in decreased
ROS production from renal epithelial cells as shown in (Figure
2) [22]. Selective inhibition of PKCε translocation, an upstream
regulator of eNOS, may mitigate ROS-related damage involved in
kidney I/R injury.
EAVSLKPT (V1-V2):
Activated PKCε binds RACK-1 for translocation to phosphorylate
ROS-producing targets, including uncoupled eNOS and
mitochondrial ATP-sensitive K+ channels [21,22]. Panel B: PKCε
inhibitor impedes the interaction between PKCε and RACK-1 and
subsequent translocation.PKCε binds to the variable region within
the RACK-1 binding site (i.e., V1-V2 region) of PKCε to regulate
translocation to cellular proteins to phosphorylate its substrate (e.g.
eNOS) [22].DGF remains a major clinical challenge. In this study, we
investigated the cell-permeable, PKCε inhibitor, YT-001, as a
potential therapeutic to prevent I/R-induced AKI.Myr- conjugation
to the N-terminus of the peptide cargo facilitates intracellular
delivery via an anchoring mechanism and simple diffusion into the
target tissue [21]. The current study used a scrambled control peptide
(N-Myr-LSETKPAV) which would not be able to inhibit the binding
of PKCε to RACK-1 [Figure 2]. The scrambled peptide is the optimal
control because it eliminates the variables of molecular weight (MW)
and peptide binding.
The aim of this study was to investigate the role of PKCε inhibition
to mitigate kidney I/R injury by comparing the effects of YT-001
to control peptide. Kidney injury was characterized by decreased
GFR, elevated serum Cr and increased PKCε localization to kidney
epithelium in Immunohistochemistry (IHC) analysis. The expected
outcome was that YT-001 would improve indices of kidney function
and reduce PKCε localization to kidney epithelial cell membranes
compared to controls.
Materials and Methods
All animal studies have been carried out in accordance with
the Guide for the Care and Use of Laboratory Animals as adopted
and promulgated by the U.S. National Institutes of Health and were
approved by the Institution’s Animal Care and Use Committee at the
University of Alabama at Birmingham.
Chemicals and Reagents:
The scrambled control peptide is N-Myr-LSETKPAV; MW =
1054, and the active peptide is N-Myr-EAVSLKPT (YT-001); MW =
1054, both provided by Genemed Synthesis, Inc., San Antonio, TX.
Anti-PKCε antibody was used in IHC analysis of PKCε localization
in kidney I/R samples (EMD Millipore, Burlington, MA; catalog
number 06-991) as described previously [23].Animal Care:
Mice were permitted continuous access to food and water. They
were kept on a 12-hour light and 12-hour dark cycle in a temperaturecontrolled
room. Thirteen male C57BL/6J mice (25–30g) were used in
this study to limit protective effects of estrogen. To minimize pain
and distress, mice received treatment with Buprenex (0.05-0.1mg/
kg; SC) pre-incision for preoperative care. It was also administered
immediately pre-op,12 hours and 24 hours post-op. After surgery,
the mice were hydrated with 0.5 mL intraperitoneal sterile salineand placed under a warm light for recovery from anesthesia for 1-2
hours until complete recovery. The mice were observed closely for
surgical complications. Any mice with a weight loss of more than 20%
were humanely euthanized. At the end of the experiment, mice were
put under deep isoflurane anesthesia prior to harvesting tissues and
euthanized via exsanguination while still under anesthesia.
In Vivo kidney I/R:
Mice were anesthetized with ketamine/xylazine prior to the
procedure. Animals were divided into two groups (YT-001 and
scrambled peptide [control]) and under aseptic precautions, an
incision was made in the left and right loin, and the renal pedicles
were exposed, secured and clamped with a micro- serrefine vascular
clamp (Fine Science Tools) for 19 minutes to induce bilateral
ischemia (i.e. both kidneys). Complete ischemia is indicated by color
change of the kidney from red to dark purple (not pale or blanching)
in minutes. The kidneys are internalized during the 19 minutes
ischemia and the skin incision is kept moist with saline soaked gauze.
One minute before unclamping, six mice were given YT-001 (1.6 mg/
kg; approximately 20 μM serum concentration), and seven were given
scrambled control peptide via tail vein injection one minute prior to
reperfusion. At the end of ischemia, clamps were removed to allow
reperfusion, which was confirmed visually. Kidneys were returned
to the abdominal cavity in their original position. The incisions were
closed with 4-0 prolene sutures, and the animals were allowed to
recover.Creatinine (Cr) Measurement:
Blood draws were collected retro-orbitally at baseline, 24, 72, and
96 hours, and serum Cr was measured using LCMS-MS as previously
described [24,25]. Reperfusion continued until sacrifice at 96 hours.Glomerular Filtration (GFR) Measurement:
GFR was measured using methods as previously described [26].
FITC-sinistrin (40 mg/mL) in phosphate buffered saline (PBS) was
administered to mice using 0.15 mg FITC-sinistrin per gram body
weight. Mice were given 2.5% isoflurane to induce anesthesia and
maintained with 1.0–1.5% isoflurane. The mice were then placed
prone on a heating pad, and fur was removed dorsally using an electric
razor. A thin layer of depilation cream was applied to the shaved area
using a cotton swab and then removed after 1-3 minutes with warm
water. A 2.5 x 3 cm2 transdermal GFR monitor was applied to the GFR
device. The battery was then connected to the GFR device and placed
on the shaved skin over the ribs using the adhesive. The device was
secured with white tape and left untouched for 3 minutes before FITCsinistrin
injection to allow for steady background reading. The tails
of the mice were then warmed in preparation for tail vein injection.
FITC-sinistrin injections were administered with an insulin syringe
in one smooth but rapid bolus. Mice were then placed in their own
cage to recover from anesthesia. GFR measurements were recorded
for 1.5 hours before the GFR device removal. The battery was then
removed from the GFR device. The GFR device was connected to the
computer using a USB, and data were read via software as previously
described [25,27].Immunohistochemistry (IHC):
HC localization of PKCε was performed as previously described
[23]. At the end of the 96-hour reperfusion period, animals were
euthanized. Kidneys were then removed, sectioned, fixed with 4%
neutral- buffered formalin, and embedded with paraffin. Tissues
were placed on a slide warmer overnight at 55°C. Sections were
de-paraffinized, and the tissues were washed in PBS three times. 10
mM citrate buffer (0.2% sodium citrate and 0.2% tween in dH2O)
was microwaved until boiling and then poured into the slide holder.
Slides were added to the solution, covered in foil and then left to sit
for 20 minutes. Slides were then rinsed in PBS three times before
protein block was added. After 10 minutes, slides were rinsed in PBS
and the primary antibody diluted in PBS (10 μL antibody in 500 μL
PBS) was added. After 190 minutes, the slides were rinsed in PBS
3 times and HRP-conjugated goat anti-rabbit antibody was added
[28]. After 15 minutes, the slides were rinsed four times in PBS. 10
μL of 3,3’-Diaminobenzidine (DAB) chromagen was added to 200
μL of DAB substrate and swirled to mix. The solution was added to
the tissue and allowed to sit for 1-10 minutes until tissues turned
light brown. The tissues were rinsed four times in PBS, then Mayer’s
hematoxylin was added. After 4 seconds tissues were rinsed in dH2O.
Tissues were then placed in bluing solution for 30 seconds and rinsed
with dH2O again. Finally, a coverslip was placed over tissues with
aqueous mounting medium. Samples with more PKCε expression
retained more brown staining and therefore produced more positive
signals. Signal positivity was measured using Aperio Image Scope
via an algorithm designed by Core Facility at the University of
Pennsylvania. The pen tool was used to outline the area of interest.
The area was then analyzed via Positive Pixel Count v9.Statistical Analysis:
Results are reported as mean ± standard error of mean (SEM).
Student’s t-tests were used for statistical analysis. Significance was
established at the 95% confidence level (P < 0.05). Sample size is
indicated by (n=).Results
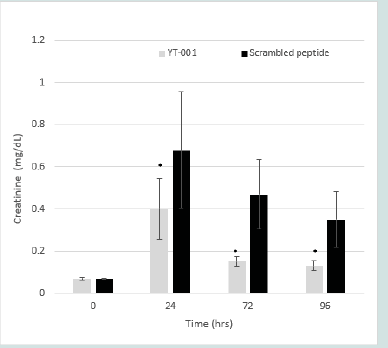
Serum Creatinine (Cr):
At baseline, serum Cr levels were the same in both treated and
control groups. Following I/R, serum Cr increased statistically in both
groups, but recovery in the YT-001 treated animals was significantly
faster and occurred to a greater extent compared to scrambled
peptides over the 96-hour period. YT-001 reduced serum Cr (from
0.07±0.01 mg/dL at 0hr, to 0.40±0.14 mg/dL at 24 hr, 0.15±0.02 mg/
dL at 72 hr, and 0.13±0.02 mg/dL at 96 hr; 24-96hr, p<0.05) relative
to control (from 0.08±0.01 mg/dL at 0 hr, to 0.94±0.20 mg/dL at 24
hr, 0.56±0.11 mg/dL at 72 hr, and 0.44±0.09 mg/dL at 96 hr). (Figure
3) depicts the serum Cr time course of YT-001 vs scrambled peptide.
Figure 3:Time course of YT-001 vs. scrambled peptide serum creatinine (Cr)
levels in murine renal ischemia-reperfusion.
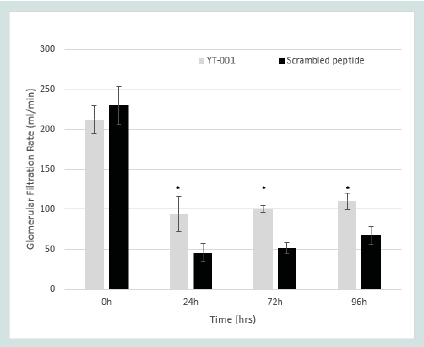
Glomerular Filtration Rate (GFR):
Prior to ischemia, GFR was the same in both groups of animals.
Following reperfusion, GFR was maintained to a significantly greater
extent in YT-001 treated animals compared to those given scrambled
peptide. GFR declined compared to baseline throughout the 96-
hour reperfusion period with greatest reduction at 24 hours in both
the YT-001 and scrambled groups. However, YT-001 maintained a
higher GFR throughout reperfusion (from 212.20±17.51 μl/min at 0
hr, to 94.18±21.62 μl/minat 24 hr, 100.24±3.87 μl/min at 72 hr, and
110.08±10.78 μl/min at 96 hr, 24-96 hr, p<0.05) compared to control
(from 230.26±23.75 μl/min at 0 hr, to 45.89±11.58 μl/min at 24 hr,
51.20±6.77 μl/min at 72 hr, and 67.59±11.27 μl/min at 96 hr). (Figure
4) displays the GFR time course of YT-001 vs scrambled peptide
(control).Serum Cr rose to 0.68 mg/dL at 24h in scrambled peptide control
mice (n=7) and to 0.40mg/dL in YT-001 mice (n=6) from baseline
values (0.07±0.007 [YT-001] vs 0.07±0.003 [scrambled peptide])
following 19-min renal ischemic injury. YT-001 significantly
attenuated serum Cr levels from 24h to 96h compared to scrambled
peptide control.
* Indicates significance compared to scrambled peptide (p < 0.05).
Glomerular Filtration Rate (GFR) in Murine Renal I/R Model.
GFR levels were calculated using FITC-Sinistrin renal clearance. 1.6
mg/kg (~20μM serum conc.) YT-001 (n=6) or scrambled peptide
(n=7) were given intravenously one-min prior to reperfusion
following 19-min renal ischemic injury. GFR was calculated at
baseline, 24h, 72h, and 96h post postischemic
injury. Scrambled peptide mice experienced a 71%
decrease in GFR (230±24μl/mL to 68±11μL/mL) following the 96 hr
reperfusion period. Conversely, YT-001 treated mice improved GFR
to 50% of baseline pre-ischemic levels (212±18μL/mL to 110±11μL/
mL) during the same period.
*Indicates significance compared to scrambled peptide (p < 0.05).
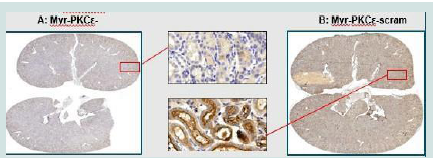
Immunohistochemistry (IHC):
IHC showed increased brown pigmentation in scrambled peptide
compared to YT-001treated tissue as seen in [Figure 5] These signals
were quantified as mean positive signals as shown in [Table 1]. The
data indicate that there was a significant decrease in localization
of PKCε in kidney exposed to YT- 001. DAB chromogen reaction
resulted in a brown precipitate indicating detection of PKCε. YT-
001 (Panel A) treatment attenuated PKCε localization in tubular
epithelium compared to scrambled peptide (Panel B). YT-001
resulted in a significant decrease in the number of positive signals
(N positive) and positivity (ratio of positive signals to all signals) in
whole-kidney samples* Indicates significance compared to scrambled peptide (p < 0.05)
in [Table 1].
Discussion
Summary of Major Findings:
This study demonstrated that i.v. administration, just prior to
reperfusion, of YT-001 (1.6 mg/kg) successfully reduced evidence of
kidney injury in an in vivo mouse model of I/R injury. In YT-001
treated animals, Cr levels increased to a significantly lower value
compared to scrambled peptide controls, and YT-001 treated
animals maintained a higher GFR compared to scrambled controls
at 24 hours, 72 hours, and 96 hours. Furthermore, IHC results
demonstrated a marked reduction in the detection of PKCε in the
YT-001treated mice, suggesting a resultant decrease in PKCε uptake
in the vascular and epithelial tissues of the treated kidneys. These
findings help delineate the way for further targeted pre-clinical
experiments to better characterize the profile and pharmacodynamics
of this peptide.Mechanism of Action Related to Various Organ Systems:
Previous literature has established the role of PKCε in activating
pro-fibrotic cytokines leading to neutrophil recruitment and
promoting the release of ROS via uncoupled eNOS activity in I/R
injuries [29-32]. PKCε expression is known to increase in acute I/R
changes to the myocardium, in stroke, and in transplanted kidneys
Figure 5:PKCε IHC staining of YT-001 and scrambled peptide treated kidney
sections following I/R (19 min/96 hrs).
Table 1:Comparison of PKCε IHC staining signal for YT-001 and scrambled
peptide control treated kidney sections following I/R.
through mitochondrial and tubular damage [33-35]. PKCε inhibitors
have been shown tohave protective effects from I/R injury on various
organs. Previous data have shown that the use of PKCε inhibitors
or PKCε deficiency reduce the myocardial infarct size and improve
overall kidney transplant function [13,35]
PKCε as a Target to Decrease I/R Injury:
As mentioned above, in previous studies, PKCε inhibition exhibits
prevention of I/R injury in an in vivo porcine heart I/R model, as
well as 90% restoration of cardiac function, and reduction of infarct
size by 70%[13]. PKCε inhibition also demonstrates reduction in
H2O2 levels in the blood in a rat hindlimb I/R model [14-15] and
in ESWL [20]. Additionally, PKCε inhibition via a trans activator
of transcription (TAT) conjugated peptide (N-YGRKKRRQRRREAVSLKPT)
effectively reduces cardiac fibrosis and inflammation
in a murine cardiac transplant model [36]. Together, these studies
demonstrate a strong basis for targeting PKCε to improve kidney I/R
injury.Peptide Conjugation:
Previous clinical studies of TAT-conjugated PKCε demonstrate
no safety or immunogenicity concerns during phase II trials
investigating the treatment of orthopedic pain and post herpetic
neuralgia [37,38]. Based on these findings, it is likely that a myrconjugated
YT-001 would have a similar safety profile. TAT is a
sequence (YGRKKRRQRRR) of primarily positive charged amino
acids (i.e. arginine [R] and lysine [K]) that facilitates intracellular
delivery of the cargo sequence via an endocytic mechanism. Whereas,
myr is a naturally occurring fatty acid that facilitates cargo delivery
via simple diffusion [21,39,40].Prevention of Polymorphonuclear (PMN) Infiltration:
It is well studied that PKCε inhibition attenuates PMN
infiltration of the heart and the resultant cytokine mediated tissue
damage [14,15,41,43]. The mechanism by which PKCε infiltrates
the heart and leads to damage suggests that the beneficial effects
of PKCε inhibition on I/R injury is likely to be translational across
organs and therefore decrease kidney damage following I/R [25,44].
I/R injury induces superoxide release from renal mitochondria
[16-19]. Superoxide, in turn, activates cytokine release, which then
activates PKCε. Once activated, PKCε stimulates both the renal
mitochondrial ATP dependent K+ channels and uncoupled eNOS,
exponentially increasing superoxide release and overall oxidative
stress [15,19,45]. Superoxide release from mitochondrial ATP
dependent K+ channels via PKCε activation is known to be a type
of preconditioning cardioprotective response prior to ischemia,
however, during reperfusion superoxide release from this source is
thought to exacerbate reperfusion injury and increase infarct size [15,16].
Thus, a PKCε inhibitor would prevent PKCε activation of both
mitochondria and uncoupled eNOS, preventing the downstream
injurious effects of ROS in I/R injury [13-15, 29-30, 46-47]Comparison of Results to Prior Studies in Regards to Kidney Function:
A prior in vivo experiment by Fuller et al. 2012 evaluated the
effect of sotrastaurin (inhibitor of independent PKC isoforms)
on I/R injuries of a transplanted kidney, which shows a significant
improvement in kidney function through reduced apoptosis and
extended renal cold preservation [48]. Both experiments were
different in several ways. First, although this experiment inhibits
a different isoform of PKC, outcomes are similar which further
emphasizes the importance and versatility of PKC inhibitors as
therapeutic targets. Second, the study by Fuller et al. used different
outcome measurements excluding Cr levels, such as urine analyses
and serial histological tissue sampling. Therefore, results are not
directly comparable due to different endpoints.Limitations:
The pre-clinical and translational nature of this study limits the
ability to extrapolate the results in a clinical setting. Further preclinical
and clinical studies (in particular, over a longer time-period)
are still needed to achieve this goal. Since only one dose was tested,
a dose-response with multiple doses will provide additional, valuable
information.Conclusion
In summary, PKCε inhibition by YT-001 had a nephroprotective
effect in in vivo I/R kidney injury. Based upon previous studies with
YT-001 in other I/R models and data from the literature, it is likely
that YT-001 inhbits ROS generation and therefore the underlying
pathology of DGF. In addition, by preserving/improving the health of
these donor kidneys, it is possible that fewer kidneys will be rejected
by transplant surgeons, thereby increasing the size of the donor
pool. PKCε inhibitors should be investigated further as a possible
therapeutic target for kidney transplant in the future. Future studies
will evaluate the long-term (9-month) protection of kidney function
in mice treated with YT-001 vs. control peptide.
Acknowledgments
Core Facility at University of Pennsylvania (IHC)
University of Alabama at Birmingham School of Medicine (in vivo kidney IR studies) Philadelphia College of Osteopathic Medicine, Department of Biomedical Sciences, Division of Research, Center for Chronic Disorders of Aging
Dr. Kerry-Anne Perkins (Young Therapeutics, LLC; Co-CSO)
Mai An Le (Research Assistant, Philadelphia College of Osteopathic Medicine)
Dr. Thomas Argentieri (Young Therapeutics, LLC; BD & Scientific Advisor)
University of Alabama at Birmingham School of Medicine (in vivo kidney IR studies) Philadelphia College of Osteopathic Medicine, Department of Biomedical Sciences, Division of Research, Center for Chronic Disorders of Aging
Dr. Kerry-Anne Perkins (Young Therapeutics, LLC; Co-CSO)
Mai An Le (Research Assistant, Philadelphia College of Osteopathic Medicine)
Dr. Thomas Argentieri (Young Therapeutics, LLC; BD & Scientific Advisor)