Download PDF
Special Issue: Cardiac Imaging: Existing Applications and Future Vision
Case Report
*Address for Correspondence: Linda E. May, MS, PhD, Foundational Sciences and Research, East Carolina University, 1851 MacGregor Downs Rd, MS 701, 3144, Greenville, NC 27834, USA, Tel: 252-737-7072; E-mail: mayl@ecu.edu Citation: May LE, Drake WB, Suminski RR, Terry MJ. Effects of Exercise during Pregnancy on Pediatric Heart Measures. J Cardiobiol. 2014;S(1): 5. Copyright © 2014 May LE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Journal of Cardiobiology | ISSN: 2332-3671 Submission: 30 August, 2014 | Accepted: 17 September, 2014 | Published: 22 September, 2014 Editors: Dr. Ernst Wellnhofer, Cardiology Specialist, Department of Internal Medicine/Cardiology, Deutsches Herzzentrum Berlin, Germany Dr. Fatih Yalcin, Fulbright Visiting Professor of Cardiobiology, Johns Hopkins University School of Medicine, USA
Effects of Exercise during Pregnancy on Pediatric Heart Measures
Linda E. May1*, William B. Drake2, Richard R. Suminski3 and Merryl J. Terry4
- 1Foundational Sciences and Research, East Carolina University, Greenville, NC, USA
- 2Pediatric Cardiology, Children’s Mercy Hospital, Kansas City, MO, USA
- 3Department of Physiology, KCUMB, Kansas City, MO, USA
- 4School of Medicine, University of Missouri, Columbia, MO, USA
*Address for Correspondence: Linda E. May, MS, PhD, Foundational Sciences and Research, East Carolina University, 1851 MacGregor Downs Rd, MS 701, 3144, Greenville, NC 27834, USA, Tel: 252-737-7072; E-mail: mayl@ecu.edu Citation: May LE, Drake WB, Suminski RR, Terry MJ. Effects of Exercise during Pregnancy on Pediatric Heart Measures. J Cardiobiol. 2014;S(1): 5. Copyright © 2014 May LE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Journal of Cardiobiology | ISSN: 2332-3671 Submission: 30 August, 2014 | Accepted: 17 September, 2014 | Published: 22 September, 2014 Editors: Dr. Ernst Wellnhofer, Cardiology Specialist, Department of Internal Medicine/Cardiology, Deutsches Herzzentrum Berlin, Germany Dr. Fatih Yalcin, Fulbright Visiting Professor of Cardiobiology, Johns Hopkins University School of Medicine, USA
Abstract
Our previous research has shown regular maternal exercise during pregnancy influences fetal cardiac development by decreasing fetal heart rate and increasing fetal heart rate variability. Whether maternal exercise promotes chronic cardiac adaptations in offspring is not known. We hypothesized that children of mothers who exercised during pregnancy would display improved cardiac function through infancy and into early childhood. An ultrasound database was queried for normal pediatric ultrasounds obtained 1 month to 7 years after birth. Mothers (n=25; 30.3 + 5.5 y of age) of cases extracted from the database completed a self-report survey to ascertain exercise behavior during the 3rd trimester. Mothers performed between 120 and 5730 minutes of exercise (M = 1449 + 14 min). Overall, minutes of exercise were not significantly correlated with any cardiac measures. However, after controlling for child’s age, minutes of exercise were negatively associated with left ventricular end systolic and diastolic dimensions (p<0.05) and left ventricular end systolic volume relative to body surface area (p<0.05). Further, minutes of exercise were positively associated with left ventricular ejection fraction (p<0.05). These results suggest exercise during late pregnancy has a significant effect on offspring heart function into early childhood independent of physiologic increases in anatomical heart size.Keywords
Exercise; Pregnancy; Pediatric; Heart; Ejection fractionIntroduction
Various types of regular physical activity improve heart health and decrease risk of cardiovascular disease. For example, physical training improves cardiac efficiency as evidenced by lower resting heart rates (HR) in trained men and women [1]. Echocardiographic findings also demonstrate cardiospecific adaptations to exercise. These findings can be seen in adults, elderly, and even in children who are regularly active. Pregnant women who engage in the current recommended amount of exercise (150 minutes/week of moderate, aerobic activity) also achieve health benefits [2,3]. With so many known benefits and cardiac adaptations to exercise in various populations, researchers have recently begun to focus on potential adaptations of the fetus in response to their mothers’ exercise. We previously reported that regular, aerobic exercise during pregnancy was associated with lower fetal HR and higher heart rate variability (HRV). HRV is a useful, noninvasive tool to assess cardiac autonomic function) at rest [4-6]. Further, we found that these cardiac parameter changes persisted into the first month of life in the offspring [7]. Reduced HRV in adults is associated with a number of cardiovascular risk factors and disease states, such as diabetes [8], obesity [9] and hypertension [10] and is a known predictor of mortality after myocardial infarct [11,12]. In the developing fetus and infant, metrics of HRV help describe the degree of integration between central and peripheral nervous systems. Moreover, the association of autonomic function and fetal behaviors are the earliest precursors to newborn behavioral regulation [13]. For example, HRV serves as an index of attention regulation in newborns [14], orientation in neonates [15], and early life information processing [16]. While it is known that maternal exercise improves fetal and newborn cardiac autonomic nervous system development, it is not known if these effects persist into early childhood. Evidence is needed to further confirm the chronic adaptive nature of fetal cardiac changes. We hypothesized that exercise during pregnancy promotes sustained improvements in measurements of childhood heart function, independent from anatomical changes.Methods
Participants TOAD software was used to query a database for normal echocardiograms as noted by pediatric cardiologists, indication for echocardiograms were murmurs found to be benign. The search was restricted to children between one day and seven years of age at the time of the exam, healthy, from a singleton pregnancy, and who had normal echocardiograms as denoted by pediatric cardiologists’ notes. In order to mail them the survey material, the names and contact information for mothers of children selected were obtained from the database. Questionnaires were sent between January 2012 and August 2012 to 200 women. Three monthly reminders were sent to women who did not respond 30 days after being sent the questionnaire. By the close of the study in December 2012, 70 mothers had returned questionnaires; however, two could not be matched with children’s data. From the remainder (n=68), 43 reported no leisure-time physical activity (LTPA) during pregnancy. While our main focus was to determine the influence of exercise during pregnancy on childhood heart measures, we focused on those cases with some type of activity and excluded cases with zero value of activity; this also normalized the distribution of maternal exercise LTPA. The remaining 25 completed questionnaires were entered into a database and then merged with their child’s ultrasound data. Physical activity questionnaire From the list of normal pediatric echocardiograms, we sent a questionnaire, a letter explaining the study, and a self-addressed return envelope to mothers. Instructions included with the questionnaire asked mothers to choose from a list and/or write in the LTPA that they participated in during pregnancy [17]. Participants provided frequency (days/week), duration (minutes/session), and intensity for each month of pregnancy and the three months prior to becoming pregnant. The Modifiable Activity Questionnaire (MAQ) was used to assess all LTPA performed during pregnancy. This questionnaire has been validated as a tool for use with pregnant and post-partum women [17]; since pregnancy represents a unique time in a woman’s life, this questionnaire is also found to be a reliable historical recall up to 6 years postpartum of physical activity during pregnancy and pregnancy weight gain [17]. Based on the researchers’ previous findings demonstrating an association between maternal duration (in minutes) of 3rd trimester LTPA and fetal/infant heart outcomes, the same measurement and procedures were used [5,6]. The primary exercise variable used in our analyses was the total duration of all LTPA performed during the 3rd trimester. Total minutes/3rd trimester was derived by multiplying the total number of sessions of third trimester LTPA and the self-reported average duration (minutes) of a session, and then adding the sum across all LTPA [5,6]. Included in this questionnaire were demographic questions about prior pregnancies, the use of substances and supplements during pregnancy, pregnancy complications, number of previous pregnancies, age, education, and pre-pregnancy height and weight. The education variable was categorical and consisted of women with and without a bachelor’s degree or higher. Pre-pregnancy body mass index (BMI) was calculated using the formula [weight (kg) ÷ height (m2)]. Ultrasound measures As a retrospective study, all heart measurements were acquired from pediatric cardiologists’ as routine patient care and thus not biased to the study question or participation. Demographic recordings were child’s age, height, and weight at time of exam. All pediatric values were indexed by body surface area (BSA) based on the Mosteller formula for children [18]. Cardiovascular measures from recorded ultrasounds were left ventricular end diastolic dimension and left ventricular end-systolic dimension. Based on these values, left ventricular end-diastolic volume, end-systolic volume, left ventricular mass, and ejection fraction were calculated. Left ventricular mass was indexed to the child’s height as well. Statistical analyses Maternal continuous demographic variable and the children’s heart measure distributions were approximately normal. The Pearson Product Moment correlation procedure was used to assess bivariate relationships between maternal variables and children’s ultrasound heart measures. Multiple regression analyses were then performed. In these analyses, pediatric heart measures were regressed (in separate models) on maternal age and minute/3rd trimester of LTPA which were the only maternal variables significantly related with at least one child heart measure in the bivariate analyses. The level of significance was set a priori at alpha < 0.05 and all statistical analyses were performed using the Statistical Package for Social Sciences (SPSS version 17.1, Chicago, 2009).Results
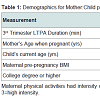
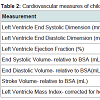
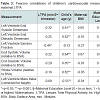
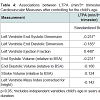
Participant demographics All women. The 25 mothers who engaged in LTPA during their 3rd trimester of pregnancy were between 30 and 42 years of age at the time of giving birth, non-smokers with no history of alcohol or drug use, and well-educated with 66.7% having at least a bachelor’s degree (Table 1). Forty-four percent had a BMI over 25 suggesting they were overweight or obese prior to becoming pregnant. Their total LTPA within the third trimester ranged between 120 and 5730 min. The children ranged in age from less than one month to seven years (Table 1). All of the pediatric heart measures examined in this study show a considerable degree of variance but were within the normal range for age (Table 2). Infant heart correlations with maternal activity (Table 3) A significant and positive correlation was found between minute/3rd trimester of LTPA and left ventricular ejection fraction (P<0.05). There were no significant correlations between maternal LTPA and left ventricular end systolic dimension, stroke volume relative to BSA, end systolic and diastolic volumes relative to BSA, and left ventricle mass index (corrected for height). The child’s age, but not maternal BMI or education level, was significantly negatively related with all the child heart measures except ejection fraction. Multiple regression analyses Provided in Table 4 are the results of the analyses where each pediatric heart measure (in separate models) was regressed on the child’s age in years and total minutes of maternal LTPA performed in the 3rd trimester. After considering the effects of the child’s age, maternal LTPA duration still accounted for a significant portion of the variance in left ventricular ejection fraction, left ventricular end systolic and diastolic dimensions and end systolic and diastolic volumes (relative to BSA). Children whose mothers engaged in more minutes of LTPA during their 3rd trimester of pregnancy had greater ejection from smaller heart dimensions and lower heart volumes, regardless of child age (p<0.05). Stroke volume relative to BSA and left ventricular mass index corrected for height were not significantly affected by maternal LTPA.Discussion
Our current study focused on determining if there was a relationship with mother’s level of physical activity while pregnant and left ventricle (LV) functional measures of her offspring. We found a significant, positive association between duration of 3rd trimester maternal LTPA and pediatric left ventricular ejection fraction, and a negative association with LV dimensions and volumes in age-adjusted data. The findings support our hypothesis that exercise during pregnancy promotes improved functional heart measurements into early childhood. Research in adults has found differences in resting LV dimensions in those who are exercise-trained relative to controls, without significant differences in ejection fraction [19,20]. Similarly, studies comparing swim-trained children (between 7-14 years of age) to nontrained controls found increased LV dimensions without changes in ejection fraction at rest [21-23]. These cardiac adaptations to exercise training are associated with lower heart rate at rest, and increased stroke volume to maintain resting cardiac output. A trained heart will exhibit lower heart rate at a given work load, increased stroke volume and cardiac output, and increased efficiency at maximal workloads. These changes are attributed to improved autonomic control as well as increased cardiac contractility, via the Frank-Starling mechanism. These physical cardiac adaptations allow the heart to function more efficiently. The left ventricular ejection fraction (LVEF) was larger in children of mothers who engaged in more LTPA during the 3rd trimester, even though these children did not have an increase in heart dimensions or volumes. It appears that children of mothers who engaged in more LTPA during the 3rd trimester had more efficient heart contractility, as evidenced by higher ejection fractions despite reduced heart dimensions and volumes. Children of mothers who engaged in lower levels of LTPA did not receive the benefit of cardiac efficiency and therefore compensated by increasing dimensions and volumes. Since many of our participants were overweight or obese prior to becoming pregnant, it is important to note that physical activity during pregnancy improves offspring heart function regardless of her fitness level prior to pregnancy. This point has significant public health implications for our increasing obesity and cardiovascular epidemic in US adults and children [24]. Although previous findings by May et al. [5,6] have shown lower heart rates in fetuses and infants exposed to maternal exercise in utero, the lack of similarities in the current findings can be explained by the timing of the exercise stimuli. For example, young male rats that exercised had no significant differences in heart weights, capillary:fiber ratio, or diffusion distance of the heart muscle relative to control rats [25]. However, male rats exposed to maternal exercise in utero have similar heart weight (absolute and relative), but significantly higher capillary:fiber ratio and decreased diffusion distance [26]. Based on prenatal and postnatal exposure to exercise, Parizkova [27] suggests prenatal exposure has a greater impact than postnatal on the microstructure of the cardiac muscle of offspring. This is relevant because it’s showing that our findings are congruent with existing literature [25-27]. Our paper discusses findings relevant to changes in the structure of the heart (LVEF), and so this responds to changes in microstructure and other ‘micro’ changes in the heart. Additionally, the effects of physical activity vary in different periods of life, and are not the same in individual tissues [28-30]. In the developing organism, physical activity tends to coincide with periods of growth. It is easier to induce structural and physiologic change in an organism that is actively growing and developing, as the cells are more sensitive to surrounding stimuli [31,32]. The age of our sample population is by design quite young and well within this developmental phase. With this in mind, our findings are most likely due to the active growth of the developing cardiac myocytes, rather than a change in the myocardium as a whole [31-35]. This current data combined with our previous findings of a lower heart rate in fetuses and infants exposed to maternal exercise further supports the Barker hypothesis of prenatal programming. These findings have exciting health implications. Exercise-related benefits generally are present only with continual participation in activity, whereas our current data suggest that prenatal exposure to maternal exercise may have lasting cardiovascular health implications independent of the child’s participation in physical activity. Therefore, regardless of the whether the child participates in physical activity or not, they may have long-term cardioprotection from prenatal exposure to exercise. Primary care providers frequently recommend exercise to their patients for a myriad of reasons, with varying levels of patient compliance. One recommended model for improving compliance involves utilizing motivational interviewing and recognizing stages of change [36]. Patients who are not currently exercising are often in the “precontemplation” stage of change, and educating the patient about the benefits of a healthy lifestyle can be beneficial in motivating the patient to begin exercise [37]. While some mothers are aware of the benefits of physical activity for their own health, the added benefit to the fetus is not taught. This information may increase the desire and compliance of physical activity in pregnant women. Additionally, the benefit of maternal exercise to the fetus included mothers who were overweight or obese; this could encourage mothers who believe that exercise would only be beneficial if they achieved a normal BMI. Lastly, the average amount of LTPA performed was below the recommended national guidelines, but still had a significant impact on childhood cardiovascular function [38]. This too will likely encourage mothers who may have thought an exercise regimen to be beyond their ability or time constraints.Limitations
The results of this study should be interpreted with regards to its limitations. Although the modifiable physical activity questionnaire has been validated for assessing physical activity in pregnant women [39] multiple years after the birth of the child, questionnaires by nature are vulnerable to recall bias that could influence estimations of physical activity levels [40]. Additionally, we did have a mother or a seven-year old participate in the study; although, this is a year older than the validation of the questionnaire, the findings did not differ when the child’s data was excluded. Second, the population of women was fairly small and educated, however, since the sample was taken from a large metropolitan area, the participants do represent a diverse background. Third, there was a wide range of reported activity levels among the active women, and the average of this population fell below the recommended activity guidelines. With this in mind, further analyses will be done with the very sedentary group of women during pregnancy and this relationship with pediatric heart function. Finally, although this was not a randomized controlled trial, the secondary analysis adjusting for maternal factors and pediatric age strengthen confidence in the current findings.Summary
Although the average intensity and time is less than the recommended guidelines by the ACOG (American Congress of Obstetrics and Gynecology website), these results are exciting and suggest physical activity during pregnancy is correlated with improved heart function of children. Though further research is warranted, these findings support the prenatal programming hypothesis and warrant further controlled study. Given the current data regarding the benefits of physical activity during pregnancy, it is essential to develop focused health initiatives targeting the gestational period. In light of the fact that cardiovascular disease is the leading cause of death of Americans, research should be done to determine if maternal exercise has a long-term cardioprotective function in children.References
- Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, et al. (2009) The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health 99: 921-928.
- Melzer K, Schutz Y, Boulvain M, Kayser B (2010) Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Med 40: 493-507.
- Exercise During Pregnancy, in American Congress of Obstetricians and Gynecologists, ACOG, Editor 2009, ACOG: Washington, DC.
- Gustafson KM, Allen JJ, Yeh HW, May LE (2011) Characterization of the fetal diaphragmatic magnetomyogram and the effect of breathing movements on cardiac metrics of rate and variability. Early Hum Dev 87: 467-475.
- May LE, Glaros A, Yeh HW, Clapp JF 3rd, Gustafson KM (2010) Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev 86: 213-217.
- May LE, Suminski RR, Langaker MD, Yeh HW, Gustafson KM (2012) Regular maternal exercise dose and fetal heart outcome. Med Sci Sports Exerc 44: 1252-1258.
- May L, Scholtz SA, Suminski RR, Gustafson KM (2014) Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev 90: 33-38.
- Fakhrzadeh H, Yamini-Sharif A, Sharifi F, Tajalizadekhoob Y, Mirarefin M, et al. (2012) Cardiac autonomic neuropathy measured by heart rate variability and markers of subclinical atherosclerosis in early type 2 diabetes. ISRN Endocrinol 2012: 168264.
- Skrapari I, Tentolouris N, Perrea D, Bakoyiannis C, Papazafiropoulou A, et al. (2007) Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring) 15: 1685-1693.
- Maule S, Rabbia F, Perni V, Tosello F, Bisbocci D, et al. (2008) Prolonged QT interval and reduced heart rate variability in patients with uncomplicated essential hypertension. Hypertens Res 31: 2003-2010.
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ (1987) Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256-262.
- Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, et al. (1992) Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. Am J Cardiol 69: 891-898.
- Porges SW, Furman SA (2011) The early development of the autonomic nervous system provides a neural platform for social behavior: A polyvagal perspective. Infant Child Dev 20: 106-118.
- Arditi H, Feldman R, Eidelman AI (2006) Effects of human contact and vagal regulation on pain reactivity and visual attention in newborns. Dev Psychobiol 48: 561-573.
- Feldman R, Eidelman AI (2006) Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature infants. Pediatrics 118: e869-878.
- Bornstein MH, Suess PE (2000) Physiological self-regulation and information processing in infancy: cardiac vagal tone and habituation. Child Dev 71: 273-287.
- Bauer PW, Broman CL, Pivarnik JM (2010) Exercise and pregnancy knowledge among healthcare providers. J Womens Health (Larchmt) 19: 335-341.
- Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317: 1098.
- Findlay IN, Taylor RS, Dargie HJ, Grant S, Pettigrew AR, et al. (1987) Cardiovascular effects of training for a marathon run in unfit middle aged men. Br Med J (Clin Res Ed) 295: 521-524.
- Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE (2000) The athlete's heart. A meta-analysis of cardiac structure and function. Circulation 101: 336-344.
- Medved R, Fabecic-Sabadi V, Medved V (1986) Echocardiographic findings in children participating in swimming training. Int J Sports Med 7: 94-99.
- Ozer S, Cil E, Baltaci G, Ergun N, Ozme S (1994) Left ventricular structure and function by echocardiography in childhood swimmers. Jpn Heart J 35: 295-300.
- Obert P, Stecken F, Courteix D, Lecoq AM, Guenon P (1998) Effect of long-term intensive endurance training on left ventricular structure and diastolic function in prepubertal children. Int J Sports Med 19: 149-154.
- (2014) White is Childhood Obesity? American Heart Association.
- Parizkova J, Wachtlova M, Soukupova M (1972) The impact of different motor activity on body composition, density of capillaries and fibers in the heart and soleus muscles, and cell's migration in vitro in male rats. Int Z Angew Physiol 30: 207-216.
- Parizkova J (1975) Impact of daily work-load during pregnancy on the microstructure of the rat heart in male offspring. Eur J Appl Physiol Occup Physiol 34: 323-326.
- Parizkova J (1978) The impact of daily work load during pregnancy and/or postnatal life on the heart microstructure of rat male offspring. Basic Res Cardiol 73: 433-441.
- Bloor CM, Pasyk S, Leon AS (1970) Interaction of age and exercise on organ and cellular development. Am J Pathol 58: 185-199.
- Parizkova J, Stankova L (1964) Influence of Physical Activity on a Treadmill on the Metabolism of Adipose Tissue in Rats. Br J Nutr 18: 325-332.
- Tomanek RJ (1970) Effects of age and exercise on the extent of the myocardial capillary bed. Anat Rec 167: 55-62.
- Jacot JG, Kita-Matsuo H, Wei KA, Chen HS, Omens JH, et al. (2010) Cardiac myocyte force development during differentiation and maturation. Ann N Y Acad Sci 1188: 121-127.
- Jacot JG, Martin JC, Hunt DL (2010) Mechanobiology of cardiomyocyte development. J Biomech 43: 93-98.
- Mollova M, Bersell K, Walsh S, Savla J, Das LT, et al. (2013) Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A 110: 1446-1451.
- Cai B, Mu X, Gong D, Jiang S, Li J, et al. (2011) Difference of sodium currents between pediatric and adult human atrial myocytes: evidence for developmental changes of sodium channels. Int J Biol Sci 7: 708-714.
- de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, et al. (1995) Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25: 1056-1062.
- Scales R, Miller JH (2003) Motivational techniques for improving compliance with an exercise program: skills for primary care clinicians. Curr Sports Med Rep 2: 166-172.
- Crookham J (2013) A guide to exercise prescription. Prim Care 40: 801-820.
- Services, U.S.D.o.H.a.H. (2007) 2008 Physical Activity Guideliens for Americans. 1st ed, Washington, DC: U.S. Department of Health and Human Services. 61.
- Cramp AG, Bray SR (2009) A prospective examination of exercise and barrier self-efficacy to engage in leisure-time physical activity during pregnancy. Ann Behav Med 37: 325-334.
- Saelens BE, Sallis JF, Black JB, Chen D (2003) Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health 93: 1552-1558.