Journal of Clinical and Investigative Dermatology
Download PDF
Research article
Evaluation of the Roleof Interleukin-33 in the Pathogenesis and Grade of the Severity of Atopic Dermatitis
Mohamed I. Metwalli1, Fathia M. Khattab1*, Elsayed G.Khater1, Naglaa A. Khalifa2
- 1Department of Dermatology, Faculty of Medicine, Zagazig University, Egypt
- 2Department of Clinical Pathology, Faculty of Medicine, Zagazig University, Egypt
*Address for Correspondence: Fathia M. Khattab, Department of Dermatology, Faculty of Medicine, ZagazigUniversity, Egypt, E-mail:fathiakhattab@yahoo.com
Citation: Metwalli MI, Khattab FM, Khater EG, Khalifa NA. Evaluation of the Role of Interleukin-33 in the Pathogenesis and Grade of the Severity of Atopic Dermatitis. J Clin Investigat Dermatol. 2017;5(2): 5.
Journal of Clinical & InvestigativeDermatology| ISSN: 2373-1044 | Volume: 5, Issue: 2
Submission: 23, October, 2017| Accepted: 12, December, 2017 | Published: 22, December, 2017
Submission: 23, October, 2017| Accepted: 12, December, 2017 | Published: 22, December, 2017
Abstract
Background:
Atopic Dermatitis (AD) is defined as a chronicinflammatory disease characterized by outbreaks of marked xerosis and intense sometimes intractable pruritus. IL-33 is defined as: A member of IL-1 family of cytokines, binds to its plasma membrane receptor, heterodimeric complex consisted of membrane-bound trans membrane ST2 (ST2L) and IL-1 Receptor (IL-1R) accessory protein, inducing nuclear factor (NF-KB) and in Mitogen Activated Protein Kinase (MAPK) activation. IL-33 can be defined also as a novel member of IL-1 family has been implicated in several inflammatory and autoimmune diseases.
Objectives:
To assess the association between level of IL33 in AD. Patients and Methods: In this study subjects were divided into two groups: Group A: Consists of twenty two patients with clinically proved AD were enrolled in this study. Patients were randomly divided into three groups according to severity depending on SCORAD: Group 1, Group 2 and Group 3. Group B: Consists of twenty two healthy subjects as control.
Results:
IL-33 is significantly high in atopic and correlated with severity of the disease. This will explain the role of IL-33 in the pathogenesis of atopy. IL-33 was statically higher in patents (p=0.000) in patients than in healthy individuals.
Conclusion:
IL-33 could play a role in the inflammatory reaction in AD.
Keywords
Interleukin 33; Atopic dermatitis
Introduction
Atopic Dermatitis (AD) is a chronic inflammatory skin disease affecting about 10 million people worldwide. A majority of AD patients’ exhibit hyper production of total IgE and most of them have allergen-specific IgE in the sera [1]. Classical T-helper type 2 (Th2) cytokines such as IL-4, IL-5 and IL-13 are expressed in the acute ezcematous lesions whereas in chronic lesions IFN-γ-producing Th1 cells are dominant [2]. In addition to classical Th2 cytokines, new tissue-derived cytokines such as TSLP and IL-33 have been suggested to have a significant role in AD [3]. In the skin lesion, signals from barrier disruption, allergens and microbial colonization are integrated and transmitted to diverse immune cells which initiate and maintain the skin [4,5].
IL-33 is a described cytokine that belongs to the IL-1 family and is linked to Th2-type immune responses [6]. IL-33 is expressed by cells of barrier tissues (skin, gut and lung) and is known to activate naive and Th2 lymphocytes, mast cells and eosinophils to produce Th2-type cytokines [7]. However IL-33 is not produced by Th2 cells and its signaling pathway distinguishes from classical Th2 cytokines [8]. IL-33 signals through a membrane-bound IL-33-specific receptor ST2 (also known as IL1RL1, T1, DER4 and Fit-1) and IL-1 receptor accessory protein (IL1-RAcP) which serves as a shared co receptor [9]. The ST2 gene encodes at least three isoforms of ST2 proteins: ST2L a transmembrane form the soluble ST2 a secreted form that can erve as a decoy receptor of IL-33 and a variant ST2 [10].
Membrane-bound expression of ST2 is highest on mast cells, Th2 cells and eosinophils which are critical cells in AD. IL-33 receptor complex is activated when IL-33 binds to ST2 and IL-1RAcP and recruits the adaptor molecule MyD88 and IL-1R-associated kinase IRAK [11]. Activated receptor complex induces activation of signaling proteins, including transcription factor NF-KB and the mitogen-activated protein kinase pathway [12].
This study aims to assess the association between level of IL-33 in atopic dermatitis patients and control subjects to determine if the high level of IL-33 is associated with atopic dermatitis or not. Also to determine possible association between the level of IL-33 and severity of disease.
Patients and Methods
Patients
Twenty two patients (12 males and 10 females) with age rangedfrom 3 to 49 years and a mean age is 10 years with clinically proved AD were enrolled in this study. They were collected from the Outpatient Clinics of the Department of Dermatology and Venereology, Zagazig University Hospitals from May 2016 to May 2017.
The diagnosis in each patient was made on the basis of history and clinical presentation. Patients were divided into three groups according to severity depending on SCORAD:
Group (A)
Group 1 included 2 patients with mild AD they represent 9.1% of patients.
Group 2 included 12 patients with moderate AD they represent 54.5% of patients.
Group 3 included 8 patients with severe AD they represent 36.4% of patients.
Group (B) control subjects
Twenty two healthy subjects (13 males and 9 females) were included as controls age their range from 4 to 36 years with a median age is 14.5 years with clinically proven healthy free of any atopic manifestations (atopic dermatitis, asthmatic bronchitis, rhinitis, conjunctivitis and any other inflammatory diseases such as RA, AS, SLE,…..)
Exclusion criteria
• Patients with other inflammatory diseases.
• Patients taking corticosteroid treatment during the previous 3 months.
• Patients aged less than 3 years.
• Patients with active viral, bacterial or fungal infections.
Methods
• Complete history taking
• General examination of body systems.
• Detailed dermatological examination.
Severity of AD was evaluated according to SCORAD index [13].• Routine laboratory examination including complete blood count (CBC), urine and stool analysis.
• Photographs of AD lesions of all patients.
• Evaluation of IL33 level using ELISA kits.
The IL-33concentrations in diluted serum was measured by sandwich ELISA. Briefly 4 μg/ml of monoclonal capture antibody (R&D Systems, Minneapolis, MN, USA) was added to a 96-well plate (Nunc, Rochester, NY, USA) and the plate was incubated for 2 hr at room temperature. The plate was then incubated in blocking solution comprising phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.05% Tween 20 for 2 hr at room temperature. All sera and was diluted 1:2 by using PBS containing 1% bovine serum albumin and 0.05% Tween 20. And standard curves were also diluted with PBS containing 1% bovine serum albumin and 0.05% Tween 20.
The test samples and standard recombinant IL-33 (R&D Systems) were added to the plates and the plate was incubated for 2 hr at room temperature. The plate was washed four times with PBS containing Tween 20, 200 ng/ml of biotinylated detection monoclonal antibodies (R&D Systems) was added and the plate was incubated for 2 hr at room temperature. The plate was washed, streptavidin-alkalinephosphatase (Sigma-Aldrich, St Louis, MO, USA; diluted 1:2,000) was added and the reaction was allowed to proceed for 2 hr at room temperature. The plate was washed four times and 1 mg/ml of p-nitrophenyl phosphate dissolved in diethanolamine (both fromSigma-Aldrich) was added to induce the color reaction which was stopped by adding 50 μL of 1 N NaOH. The optical density at 405 nm was measured on an automated microplate reader (VERSA Max, Molecular Devices, Palo Alto, CA, USA). A standard curve was drawn by plotting optical density versus the log of the concentrations of IL- 33 level was measured using the DuoSet ELISA kit (R&D Systems) following the manufacturer’s protocol.
Statistical analysis
Quantitative data are expressed by median and comparison between 2 medians by Mann Whitney U test.
Comparison of several medians by kruskal-Wallis H test. Spearman rank correlation coefficient (r) for assisting relation between 2 quantitative variables.
Qualitative data are expressed as number and percent association done by Chi Square test.
P value less than 0.05 is considered significant.
Results
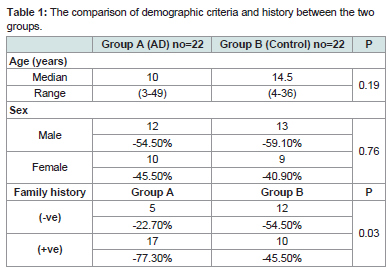
Demographic, history, clinical and laboratory criteria of the studied patients are demonstrated in (Table 1). Both patients and controls are matched regarding age (p=0.19) and sex (p=0.76). There is no significant difference between the two groups regarding family history. However patients with AD had (+ve) family history (17=77.3% of patients) more than controls as (+ve) family history resembles (10 =45.5% of controls).
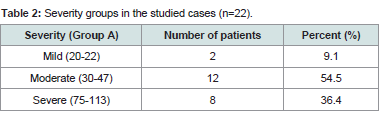
Shows that moderate form of the disease constitutes the majority of cases (54.5 %) followed by severe and mild forms (36.4 % and 9.1 % respectively) (Table 2).
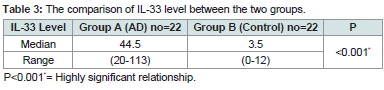
Shows a highly significant difference in the level of IL-33 between patients with median (44.5 ng/ml), range (20-113) and controls with median (3.5) range (0-12). Highly significant relationship between level of IL33 in atopic group (P) (Table 3).
The level of IL-33 is not significantly affected by sex. However its range in male patients (20-113) more than in female patients (22-90) and presence of (+ve) family history also not affect its level.
There is no statistical significant correlation between IL-33 serum level and age but there is a high statistical significant difference between it l and duration of AD in the patients
There is no statistical significant correlation between IL-33 serum level and association of other atopy.
Also there is a high statistical significant correlation between it IL-33 serum level and severity degrees in atopic dermatitis with highest median (86.5) and highest range (75-113).
There is no statistical significant correlation between IL-33 serum level and age.
The level of IL-33 is not significantly affected by sex. IL-33 level is higher in females and in control with (+ve) family history but with no statistical significance as p=0.86 and 0.06 consecutively, however its range in male patients (0.00-0.12).
Discussion
Atopic Dermatitis is a chronic, relapsing and intensely pruritic inflammatory skin condition that usually affect infants, children and young adults [14].
It is characterized by outbreaks of marked xerosis and intense sometimes intractable pruritus [15]. AD also characterized with early onset and lifetime prevalence of approximately 20% [2].
Thus the non-IgE associated (formerly intrinsic or atopiform dermatitis) form has to be distinguished from IgE mediated (formerly extrinsic) form. However some authors propagated the concept of 2 distinct forms, atopiform dermatitis versus AD the non-IgE associated form may present a transitional phase of IgE associated form [2].
Atopic dermatitis is the most common skin disease in childhood with a prevalence of 10-15% at the time of school entry [16].
Atopic Dermatitis affects 20% of children and almost 3% of adults and is associated with considerable impairment of quality if life for both patients and their families while the condition resolves spontaneously after puberty in over 75% of cases but also can persist into adulthood [15].
Cutaneous Lymphocytic Antigen (CLA) expressing CD8 T cells have been known to play an important role in the pathogenesis of AD [17].
Various factors contribute to a high-Th2/low-Th1 cell environment with regards to the predominance of T cells in AD skin [6].
Interleukin -33 is one of IL-1 family and has been described to function as an alermine, IL-33is expressed in tissues with predominant barrier function and released up on cell death with activation of several components of immune system this is accordance with Pushparaj et al. [18].
Furthermore IL-33 can also act as a chemoattractant for Th2 cells, IL-33 moreover induce expression of Th2 cytokines IL-5 and IL-13 in vitro as well as increased blood eosinophils and serum mmunoglobulins when derived in vivo [11].
IL-33 can also induce the activation and maturation of human mast cells. Th2 cells are important players in coetaneous allergic inflammation, an integral part of AD. Th2 cytokines can be secreted by basophiles, eosinophils and mast cells. Basophiles have been detected in AD skin lesions and eosinophils recruitment into the skin is characteristic of AD [14].
The expression of IL-33 and ST2 were found to be increased in the skin of patients with AD after a challenge with an allergen or staphylococcal enterotoxins B. TNF-a dose dependently induced IL-33 gene expression in healthy human skin. Furthermore the expression of IL-33is enhanced in psoriatic skin and reduced by an anti-TNF a therapy in IL-33 transgenic mice which show skin selective IL-33 gene expression various AD-like skin symptoms such a spontaneous itching, eosinophil infiltration and mast cell hyperplasia were induced [20].
In children the flexures of the knees and elbows the lesions appear as red scaly areas or crusting and weeping in the acute stages and thickened (lichenified) and secondary infection as seen in infants may also occur [21].
Less than 10% the eczema continues into adulthood. Atopic eczema may present for the first time in adult life. In adults, lichenified patches on the flexures of the knees and elbows may occur [22].
Scoring of AD according to the SCORAD index which consists of the interpretation of the extent of the disorder (A: according to the rule of nines, 20% of the score). Subjective symptoms (C: itch, sleeplessness; 20% of the score). The intensity composed of six items (B: erythema, edema/ papules, effect of scratching, oozing/ crust formation, li chenification and dryness, 60% of the score each item has four grades: 0,1,2,3 [23].
The most important complications of AD are due to secondary bacterial and viral infections. The course of this complication may be severe with high fever and wide spread eruptions [24]. Also neurological symptoms as paresthesia may occur in AD and social withdrawal occurs [25].
The aim of this study is to assess the association between level of IL33 in AD patients and control subjects to determine that of the high evel of IL33 is associated with AD or not also to determine possible association between the level of IL33 and severity of the disease.
In our study: Age of patients ranged from 3 to 49 years and this agree with Abdel Hay et al. Who act on more or less nearly in the same age group but with more early age of onset in our study [12]. Also according to sex of patients male to female ratio 1.2 to 1 and this also similar to Abdel Hay et al. Study in which male to female ratio was 1.5 to 1 [12].
Personal history of other atopic manifestations in patient groups in our study is 77.3% (17) patients and also (+ve) family history was found in 77.3% patients [17]. While Abdel Hay et al. found other atopic manifestations like asthma in 30% (6 patients) of patients and also +ve family history in 30% of patients also [14]. This may be ttributed to the difference in severity and early onset in our patients.
No significant relationship between IL-33 with age, gender, history of asthma and other atopy in patient group in our study show that agree with Tamagawa-Mineoka et al. [26].
In this study IL-33 was statically higher in patients (p=0.000) than in healthy individuals. Sakashita et al. was in agreement with our study as the serum level of IL-33was studied in a large group of patients suffering from allergic rhinitis sensitive to Japanese cedar the serum level of the IL-33 was higher than in the controls [27].
Also our results were also in agreement with Matsuda et al who found that IL-33 in allergic conjunctivitis not in the control conjunctivae the authors also confirmed mature IL-33 protein expression in ocular resident cells by western blot analysis [28].
Tamagawa-Mineoka et al. Found that serum levels of IL-33 are significantly elevated in patients with AD compared with healthy control subjects (P<0.01) and also serum IL-33 level was significantly associated with EASI score [26].
In this study, IL-33 is significantly high in atopic and correlated with severity of the disease. This will explain the role of IL-33 in the pathogenesis of atopy.
Severity groups in the studied cases in our study mild cases were 9.1% (2 patients) moderate cases 54.5% (12 patients) and severe cases 36.4% (8 patients) This differ from Abdel Hay et al. who act on group of patients with mild cases 40% (8 patients) mild cases 25% (5 patients) and severe cases 35% (7 patients) [12].
According to score of severity as value=0.000 this mean highly significant relation between IL-33 level and grade of severity. This is in accordance with Tamagawa-Mineoka et al. who found score of severity is the only factor correlate with level of IL-33 as ( P= 0.005) this agree with our study [26].
Our study is accordance with Abdel Hay et al. who found that IL-33 was statistically high (p<0.001) detected in AD patients and correlated with severity of the disease (p<0.011) [12].
However IL-33 level in study of Abdel Hay et al. IL-33 detected in all patients in range of 194.5-516.3 pg/ml with a mean of 292.5 ± 89.68 pg/ml higher than in our study with range of 22-113 pg/ml with median of 44.5. This may be attributed to the difference in the method of estimation as in our study we estimate serum level of IL-33 in AD but in the other study IL-33 was estimated in tissues.
On the other hand our results are in agreement with Gluck et al. who found that elevated levels of IL-33 in sera of intermittent allergic rhinitis and that the serum level of IL-33 correlated with the disease severity [29].
Gluck et al. found that the IL-33 is a marker of intermittent allergic rhinitis severity and the median value of IL-33 (28.5) and its range (15-123) is near to the values in our study as median value of IL-33 (44.5) and its range (20-113) this is attributed to the same method of measurement in sera [29].
In the end: We found elevated levels of IL-33 in sera of AD patients and that the serum level of IL-33 correlated with the disease severity. Our results suggested that IL-33 could play a role in the inflammatory reaction in AD.
Conclusion
We found elevated levels of IL-33 in sera of AD patients and that the serum level of IL-33 correlated with the disease severity. Our results suggested that IL-33 could play a role in the inflammatory reaction in AD (IL-33 involved in pathogens is of AD) The results may provide new insight into the pathophysiology of the disease and into novel therapeutic targets, however this finding should be confirmed by further studies with large scales of patients.
References
- Bieber T (2010) Atopic dermatitis. Ann Dermatol 22: 125-137.
- Thomsen SF (2014) Atopic dermatitis: Natural history, diagnosis and treatment. ISRN Allergy 2014: 354250.
- Du HY, Fu HY, Li DN, Qiao Y, Wang QW, et al. (2016) The expression and regulation of interleukin-33 in human epidermal keratinocytes: A new mediator of atopic dermatitis and its possible signaling pathway. J Interferon Cytokine Res 36: 552-562.
- Ellis CN, Mancini AJ, Paller AS, Simpson EL, Eichenfield LF (2012) Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg 31(3 Suppl): 18-22.
- Eichenfield LF, Ellis CN, Mancini AJ, Paller AS, Simpson EL (2012) Atopic dermatitis: Epidemiology and pathogensis update. Semin Cutan Med Sug 31(3 Suppl): S3-5.
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, et al. (2010) IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A 107: 18581-18586..
- Liew FY, Pitman NI, McInnes IB (2010) Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol 10: 103-110.
- Brandt EB, Sivaprasad U (2011) Th2 cytokines and atopic dermatitis. J Clin Cell Immunol 2: 110.
- Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, et al. (2009) IL-33-activated dendritic cells induce and atypical TH2-type resonse. J Allergy clin Immunol 123: 1047-1054.
- Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, et al. (2008) IL-33 induces antigen-specific IL-5+T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 181: 4780-4790.
- Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, et al. (2007) IL-33 is a chemoattractant for human Th2 cell. Eur J Immunol 37: 2779-2786.
- Abdel Hay RM, Ibrahim NF, Metwally D, Rashed LA (2013) The role of Interleukin-1β and interleukin-33 in atopic dermatitis. Our Dermtol Online 4: 11-14.
- Eurpean Task Force on Atopic Dermatitis (ETFAD) (1993) Severitg scoring atopic dermatitis: The SCORAD index. Dermatolog 186: 23-31.
- Chan S, Burrows N (2009) Atopic dermatitis. Common dermatosis 37: 242-245.
- Garnacho-Saucedo G, Salido-Vallejo R, Moreno-Giménez JC (2013) Atopic dermatitis: Update and proposed management algorithm. Actas Dermosifiliogr 104: 4-16.
- Werfel T, Schwerk N, Hansen G, Kapp A (2014) The diagnosis and graded therapy of atopic dermatitis. Dtsh Arztebl Int 111: 509-520.
- Zhang BX, Lyu JC, Liu HB, Feng DQ, Zhang DC, et al. (2015) Attenuation of peripheral regulatory T-cell suppression of skin homing CD8+Tcells in atopic dermatitis. Yonsei Med J 56: 196-203.
- Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, et al. (2009) Retraction. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A 109: 13877
- Pilus A, Owczarek W, Nawrocka A (2011) The role of IL-33 and IL-13 in atopic dermatitis. Allergol Clin Immunol 17: 1-2.
- Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, et al. (2012) IL-33/ST2 axis in inflammation an immunopathology. Immunol Res 52: 89-99.
- Bieber T (2008) Atopic dermatitis. N Engl J Med 358: 1483-1494.
- 22.Ellis CN, Mancini AJ, Paller AS, Simpson EL, Eichenfield LF (2012) Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg 31(3 Suppl): S18-22.
- Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB (2007) Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol 157: 645-648.
- Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, et al. (2009) Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol 124: 260-269.
- 25.Katayama I, Aihara M, Ohya Y, Saeki H, Shimojo N, et al. (2017) Japanese guideline for atopic dermatitis 2017. Allergol Int 66: 230-247.
- Tamagawa-Mineoka R, Okuzawa Y, Masuda K, Katoh N (2014) Increased serum level of interleukin 33 in patients with atopic dermatitis. J Am Acad Dermatol 70: 882-888.
- Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, et al. (2008) Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy 38: 1875-1881.
- Matsuda A, Okayama Y, Terai N, Yokoi N, Ebihara N, et al. (2009) The role of interleukin-33 in chronic allergic conjunctivitis. Invest Ophthalmol Vis Sci 50: 4646-4652.
- Glück J, Rymarczyk B, Rogala B (2012) Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Inflamm Res 61: 574-550.