Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Diagnostic Value of Dermoscopic Findings of Hair and Scalp in Cicatricial Alopecia
Kandil AH1, Abdelshafy AS1*and El-Kashishi KA2
- 1Department of Dermatology and Venereology, Zagazig University, Egypt
- 2Department of Pathology, Zagazig University, Egypt
*Address for Correspondence: Abdelshafy AS, Department of Dermatology and Venereology, Zagazig University, Zagazig, Egypt, Tel: 201008364064; E-mail: ahmed_said3007@yahoo.com
Citation: Kandil AH, Abdelshafy AS, El-Kashishi KA. Diagnostic Value of Dermoscopic Findings of Hair and Scalp in Cicatricial Alopecia. J Clin InvestigatDermatol. 2018;6(2): 5
Copyright:© 2018 Kandil AH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of clinical and Investigative Dermatology| ISSN: 2373-1044 | Volume: 6, Issue: 2
Submission: 29 September, 2018 | Accepted: 5 November, 2018 | Published: 6 November, 2018
Abstract
Background: Cicatricial Alopecias are a group of uncommon hair loss disorders, which are characterized by permanent destruction of hair follicles. Dermoscopy is a recent enlightening tool in the dermatological diagnostic field.
Objective: To evaluate the dermoscopic findings of Cicatricial Alopecia and the possibility of utilizing dermoscopy for assessment of Cicatricial Alopecia
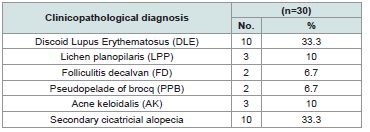
Patients and Methods: Thirty patients with Cicatricial Alopecias were the subjects of this study. They were classified according to the clinicopathological diagnosis into: Discoid lupus erythematosus 10 cases (33.3%), lichen planopilaris 3 cases (10%), folliculitis decalvans 2 cases (6.7%), pseudopelade of brocq 2 cases (6.7%), acne keloidalis 3 cases (10%) and finally other secondary causes 10 cases (33.3%). The skin lesions were examined histopathologically to confirm the diagnosis and the lesions were examined dermoscopically.
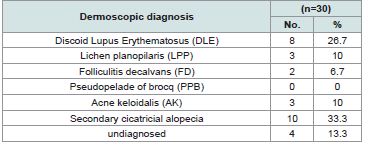
Results: The dermoscopic diagnosis of the patients proved the following diagnoses: Discoid lupus erythematosus 8 cases (26.7%), lichen planopilaris 3 cases (10%), acne keloidalis 3 cases (10%), folliculitis decalvans 2 cases (6.7%), undiagnosed 4 cases (13.3%), other secondary causes 10 cases (33.3%) and no cases diagnosed as pseudopelade of brocq.
Conclusions: The dermoscope provides a practical and useful aid for the clinical diagnosis of different types of Cicatricial Alopecia.
Keywords
Cicatricial alopecia; Dermoscopy; Hair and scalp
Introduction
Scalp alopecias are considered a broad spectrum of heterogeneous diseases and they are among the most common dermatologic disorders [1]. Alopecias are classified into non-cicatricial and cicatricial forms.Pathologically, the scar is the end point of reparative fibrosis with permanent destruction of preexisting tissue [2].
Cicatricial Alopecias (CA) are a group of uncommon inflammatoryhair loss disorders, which are characterized by permanent destructionof hair follicles. Clinically there is loss of visible follicular ostia in scarring area, with or without epidermal atrophy and histologically there is absence of pilosebaceous structures which are replaced byfibrous tract [3]. Cicatricial Alopecia may be divided into a ‘primary’ condition when the follicle is the main target of the disease process or a ‘secondary’ event when the follicle acts as an ’innocent bystander’ in the course of a disease occurring outside the follicular unit [4].
Primary Cicatricial Alopecias (PCAs) are probably the most misdiagnosed scalp disorders and frequently cause major distress for the affected patient. These scalp diseases represent trichologic emergencies because hair follicles are permanently destroyed and therefore the patient might experience disfiguration, psychological embarrassment, and lack of self-esteem. A quick and confident proof of diagnosis and aggressive treatment in the case of active disease are [5].
In 2001, the North American Hair Research Society (NAHRS) put forward a “proposed working classification of the primary Cicatricial Alopecias” based on the predominant type of inflammatory cell component [6]. Four groups have been considered as follows: lymphocytic, neutrophilic, mixed, and non-specific. LymphocyticCAs include Chronic Cutaneous Lupus Erythematosus (CCLE), Lichen Planopilaris (LPP), Pseudopelade of Brocq (PPB), central Centrifugal Cicatricial Alopecia (CCCA), Alopecia Mucinosa (AM), Keratosis Follicular is Spinulosa Decalvans (KFSD). Neutrophilic CAs includes Folliculitis Decalvans (FD) and Dissecting Cellulitis (DC). Mixed CAs includes Acne Keloidalis Nuchae (AKN) and acnenecroticavarioliformis.
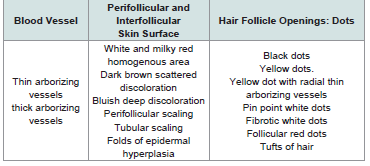
Trichoscopy is dermoscopy of the hair and scalp. This is a noninvasive, in office technique that can be performed with a handheld dermoscope or a digital videodermoscopy system. Trichoscopy helps for magnified observation of four components|: (1) hair shafts, (2) hair follicle openings, (3) the perifollicular epidermis, and (4) blood vessels. Abnormalities in the appearance of these 4 structural components of the scalp aid in the differential diagnosis of hair loss[7].
The aim of the study was to evaluate the dermoscopic findings of Cicatricial Alopecia and the possibility of utilizing dermoscopy for assessment of Cicatricial Alopecia.
Patients and Methods
Patients
This study included thirty patients with different clinical varieties of CA. The patients were selected from the Outpatient Clinic of Dermatology and Venereology Department, Zagazig University Hospitals, during the period from January 2015 to August 2015 after obtaining the approval of the research ethics committee of the hospital (code no. ZUIRB#1743-14-12-0-2-14) and written informed consent from each participant.
The inclusion criteria include patients with hair loss, diagnosed clinically as Cicatricial Alopecia established by histopathological examination at any age, sex, and duration. Those with any hair and scalp disorders other than Cicatricial Alopecia like alopecia are ata, seborrheic dermatitis, psoriasis and acute telogen effluvium were excluded from the study.
Methods
All Subjects in this study were subjected to complete history taking including personal history, family history of a similar condition, present history including onset, course, duration and previous therapy as well as past history of drug intake or systemic disease. General examination was done to diagnose any systemic isease. Dermatological examination was done including site, size, and texture of the skin and distribution of hair loss.
Histopathological examination
Four mm punch biopsy specimens were obtained from each patient from the edge of the lesion. The specimens were fixed in 10% neutral buffered formalin and processed for paraffin embedding. 4 m sections was obtained from paraffin blocks and stained with Haematoxylin and Eosin (Hx.&E.) stain for histopathological diagnosis.
Dermscopic examination
Dermscopic examination Lite II model, palm-sized, large 25 mm lens permits scalp visualization at a 10-fold magnification, without an interface solution. The digital camera used is (Sony Cyber-shot DSC-W710) (Sony corp, Tokyo, Japan), which offers high resolution (16, 1 megapixel) that produced imagery with a magnification of 50-fold via its 5-fold optical zoom lens, which helped us in magnifying the lesion viewed by the dermoscope. It has a large screen (2.7 inch LCD) and rechargeable lithium-ion battery. Photos were obtained by the digital camera. Several different images at 10- to50-fold magnifications were taken, and evaluated in accordance with the checklist of dermoscopic features presented in Table 1.
Statistical analysis
All data obtained were analyzed with SPSS version 15 (IBM Co., New York, New York, USA). Data were summarized using mean ±SD. Comparison of quantitative data was made using Student’s t-test. Comparison between groups was made using the X2-test and Fisher’s exact test for qualitative variables. Statistical significance was determined at a level of P less than or equal to 0.05.The sensitivity of a clinical test refers to the ability of the test to correctly identify those patients with the disease.
Results
The studied patients were 13 males and 17 females with their ages ranging from 1 to55 years with a mean of 34.5± 9.32 years. The cases were 20 diagnosed as primary Cicatricial Alopecia and 10 diagnosed as secondary Cicatricial Alopecia. Secondary cases of Cicatricial Alopecia were 6 cases due to burns, 2 cases due to morphea and 2 cases due to trauma.
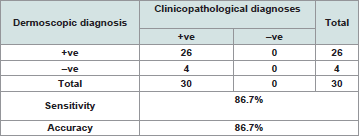
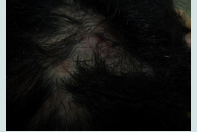
The clinicopathological diagnoses of the studied group were summarized in and in photos 1a, 2a, 3a, 4a and the dermoscopic diagnoses of the studied group were summarized in and in photos 1b, 2b, 3b and 4b (Table 2 and 3).
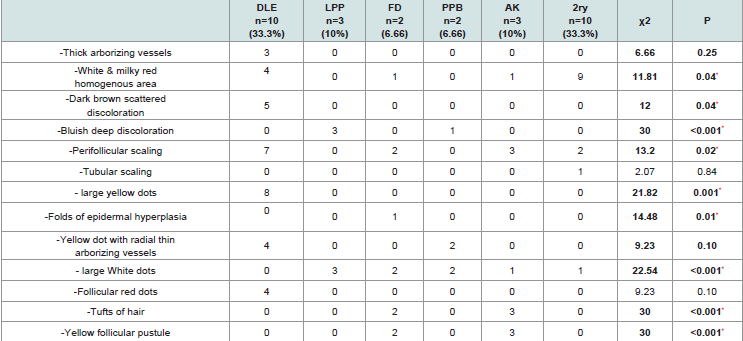
. The relation between dermoscopic findings and diagnosis of the studied group was summarized in Table 4.There was no significant difference between clinicopathological and dermoscopic diagnosis of the studied cases with p value of 0.74 and the sensitivity of dermoscope in diagnosis of Cicatricial Alopecia in comparison to clinicopathological diagnosis was 86.7% Table 5.
Table 5: Validity of dermoscope in diagnosis in comparison to histopathology as a gold standard for diagnosis.
Dermoscopic features in discoid lupus erythematosus
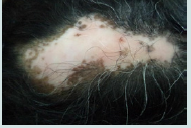
Large yellow dots, dark brown scattered discoloration, thick arborizing vessels, yellow dot with radial thin arborizing vessels, and follicular red dots were detected only in DLE. Large yellow dots and dark brown scattered discoloration were significantly more common in DLE (p<0.001) & (p=0.04) respectively (Figure 1a and Figure1b),(Figure 2a and Figure2b).
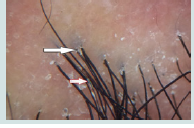
Figure 1b: Yellow dots containing thin spider vessels (arrows) in inactive discoid lupus erythematosus.
Figure 2b: Perifollicular collar scaling in lichen planopilaris by dermoscopy. Scales form silver-white tubular structures around the emerging hair shafts, usually reaching about 1-3 mm above the scalp surface (arrow).
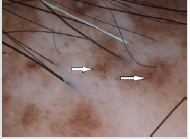
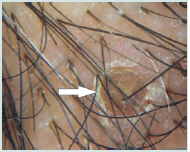
Large yellow dots were reported in 8patients (80%), dark brown scattered discoloration were reported in 5 patients (50%), yellow dot with radial thin arborizing vessels were reported in 4 patients (40%), follicular red dots were detected in 4 patients (40%), and thick arborizing vessels were reported in 3patients (30%) were reported in 50%.Tufts of hair and yellow follicular pustule weredetected in all cases of FD (100%). They were significantly common in FD (p<0.001) (Figure 3a and Figure 3b).
Figure 3b:The white arrow points to tubular scaling around three hairs emerging from a follicular unit. The red outlined arrow points to hair casts in folliculitis decalvans.
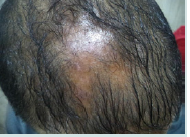
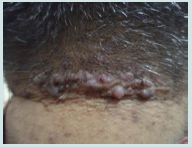
Dermoscopic features in acne keloidalis
Tufts of hair and yellow follicular pustule were detected in all patients of AK. They were significantly more common in AK (p<0.001) (Figure 4a and Figure 4b).
Discussion
Our study revealed the following characteristic dermoscopic features: In DLE, large yellow dots, dark brown scattered discoloration follicular red dots and thick arborizing vessels. Large yellow dots were seen in 80% of DLE patients and this finding was also detected by in 55% of patients, and in 70% of DLE patients by which are near to our study results [8,9]. Large yellow dots seen in DLE significantly differed from yellow dots observed in alopecia areata and androgenetic alopecia as they were larger and darker (dark-yellow to yellowbrown in color). Large yellow dots with radial, thin arborizing vessels emerging from the dot were found to be specific to DLE (Red spider in yellow dot) as it isn’t seen in other causes of CA.
Scattered dark-brown discoloration of the skin giving a “dirty” appearance was observed in active DLE, in 50% of DLE patients. This finding comes also in parallel to who reported it in 45% of DLE patients and was detected in 70% DLE patients by [8,9].
Thick arborizing vessels were found only in 30% of DLE patients of our study. This proportion of thick arborizing vessels seen in our DLE lesions is much lower than that found by who found thick arborizing vessels in almost all patients with DLE [8]. However, thick arborizing blood vessels were seen in 80% cases of DLE patients by [9]. This difference indicates that this finding isn’t an absolute criterion for diagnosis of DLE dermoscopically and may be attributed to different patient selection with different activity of the disease. Thick arborizing vessels observed in DLE appeared slightly more irregular with variable thickness in the course of a vessel which differ from those found in BCC.
Follicular red dots were found in 40% of DLE patients. This much higher than that found by who detected it in only 5% of DLE patients and by who did not find it at all found follicular red dots in active DLE and their presence correlates with dilated infundibulum surrounded by dilated vessels with pronounced red blood cell extravasation [8-10]. The presence of follicular red dots in DLE lesions is a good prognostic feature and indicates high probability of hair regrowth.
In lichen planopilaris, the most characteristic dermoscopic features are: bluish deep discoloration and large white dots. We found bluish deep discoloration in all patients with LPP this findingwas detected by in only 45% of patients [8]. This difference may be explained by long duration of LPP patients in our study. Our studyconfirms the presence of white dots in all patients with LPP and matches with the study of [11]. The classic white dots of LPP, which correspond to vertically-oriented fibrotic tracts in histopathology, may be observed in most primary Cicatricial Alopecias. They are irregular in shape, have blunt borders, and a tendency to merge and form white areas devoted of follicular opening and differ from the smaller “pinpoint” white dots, first observed in patients with dark skin and correspond to empty hair follicle openings or eccrine sweat duct openings. These “pin point” white dots are small, regularly oval, or circular shaped, sharply demarcated from the brown surrounding skin [12]. We did not find tubular perifollicular scaling, and elongated, parallel-oriented blood vessels. This may be due to end stage disease of our patients.
Dermoscopic features of pseudopelade of Brocq in our study are nonspecific including no features of inflammation and noperifollicular scaling and absent follicular openings and the skincolour is usually porcelain white. Thus, pseudopelade of Brocq is adiagnosis of exclusion both clinically and trichoscopically and thismatches with the conclusion of [12]. In folliculitis decalvans, our study revealed the following characteristic rich scopic features: yellow and large follicular pustule with an emerging hair shaft, perifollicular scaling, tuft of hair, and folds of epidermal hyperplasia. Large follicular pustule with an emerging hair shaft is a hallmark of FD present in all patients. This finding was also detected in other studies as found black dots, absent follicular openings, elongated linear blood vessels, perifollicular erythema, epidermal atrophy and cicatricial white patches and no large follicular pustule with an emerging hair shaft [8,9]. Hair tufts surrounded by starburst pattern epidermal hyperplasia described by found in all cases of FD corresponds in histopathology to hyperkeratosis, with parakeratosis overlying a hyperplastic epidermis [8]. White and milky-red areas lacking follicular openings were seen in all types of PCA. This finding was detected in most of the studies of PCA. Finally, our study revealed that there is no significant difference between histopathologic diagnosis and dermoscopic diagnosis.
Conclusion
The polarized-light handheld dermoscope attached to a digital camera provides a practical and useful aid for the clinical diagnosis of different types of Cicatricial Alopecia. Moreover, it has low cost and less invasiveness when compared to the histopathology. We recommend further studies with larger number of patients and different stages of the disease activity.
References
- KaradağKöse Ö, Güleç AT (2012) Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol 67: 206-214.
- Sellheyer K, Bergfeld WF (2006) Histopathologic evaluation of alopecias. Am J Dermatopathol 28: 236-259.
- Abraham LS, Piñeiro-Maceira J, Duque-Estrada B, Barcaui CB, Sodré CT, et al. (2010) Pinpoint white dots in the scalp: dermoscopic and histopathologic correlation. J Am Acad Dermatol 63: 721-722.
- Griffin LL, Michaelides C, Griffiths CE, Paus R, Harries MJ, et al. (2012) Primary cicatricial alopecias: a U.K. survey. Br J Dermatol 167: 694-697.
- Mubki T, Rudnicka L, Olszewska M, Shapiro J (2014) Evaluation and diagnosis of the hair loss patient: part II. Trichoscopic and laboratory evaluations. J Am Acad Dermatol 71: e1-e11.
- Otberg N (2013) Primary cicatricial alopecias. Dermatol Clin 31: 155-166.
- Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, et al. (2003) Workshop on Cicatricial Alopecia. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol 48: 103-110.
- 8.Mubki T, Rudnicka L, Olszewska M, Shapiro J (2014) Evaluation and diagnosis of the hair loss patient: part II. Trichoscopic and laboratory evaluations. J Am Acad Dermatol 71: e1-e11.
- 9.Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik O, Czuwara J, et al. (2012) Trichoscopy of cicatricial alopecia. J Drugs Dermatol 11: 753-758.
- Thakur BK, Verma S, Raphael V (2015) Clinical, trichoscopic, and histopathological features of primary cicatricial alopecias: A retrospective observational study at a tertiary care centre of North East India. Int J Trichology 7: 107-112.
- Tosti A, Torres F, Misciali C, Vincenzi C, Starace M, et al. (2009) Follicular red dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol 145: 1406-1409.
- Ross EK, Tan E, Shapiro J (2005) Update on primary cicatricial alopecias. J Am Acad Dermatol 53: 1-37.