Journal of Clinical and Investigative Dermatology
Download PDF
Case Report
Oral Lichen Planus Developing after PD-1 Inhibitor Therapy in Two Patients with Malignant Melanoma
Jain N1, Panah E1, Garfield E1, Quan V1, Shi K1, Mohan L1, Yoo S1, Chandra S2, Sosman J2 and Gerami P1*
1Department of Dermatology, Northwestern University Feinberg School of Medicine, USA.
2Division of Hematology and Oncology, Northwestern University Feinberg School of Medicine, USA.
*Address for Correspondence:
Gerami P, Northwestern University, Department of Dermatology, 676 N. St Clair St., Suite 1600Chicago, IL, USA, Tel: (312) 695-1413; Email: p-gerami@northwestern.edu
Submission: 30 May, 2019
Accepted: 19 June, 2019
Published: 28 June, 2019
Copyright: © 2019 Jain N, et al. This is an open access article distributed
under the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original work
is properly cited.
Abstract
Cutaneous adverse reactions to checkpoint inhibitor therapy commonly
include non-specific dermatitis and pruritus, though the development of
a wide range of dermatologic toxicities has been described, including
lichenoid reactions. Occasional oral mucosal involvement has been
reported, with rare erosive changes. Here, we present two cases of erosive
oral lichen planus developing after PD-1 inhibitor therapy for malignant
melanoma, with one case that later progressed to diffuse cutaneous
involvement. Oral involvement in both cases was treated with topical high
potency steroids and topical tacrolimus. This treatment regimen allowed one
patient to continue immunotherapy and the other to resume immunotherapy
after it was discontinued. These cases support erosive oral lichen planus as
a notable, serious adverse effect of PD-1 inhibitors.
Introduction
Advances in immunotherapy, including the programmed death
1 (PD-1) checkpoint inhibitors nivolumab and pembrolizumab,
have considerably changed the management of advanced malignant
melanoma. These monoclonal antibodies impede binding of PD-1
with its ligand, PD-L1, preventing T-cell apoptosis and stimulating
antitumor response [1]. Cutaneous immune-related adverse effects
from PD-1 inhibitor therapy occur frequently, with non-specific rash
and pruritus among the most commonly reported [2,3]. The extent
of their cutaneous toxicities, however, has yet to be fully identified.
Here, we present two patients with advanced malignant melanoma
who underwent adjuvant therapy with nivolumab and later presented
with erosive oral lichen planus.
Case Presentation
Case 1: A 78-year-old female with a history of Diffuse Large B-Cell
Lymphoma (DLBCL) and malignant melanoma presented to
dermatology clinic for painful erosive lesions on her lower lip
following two months of therapy with nivolumab. She was initially
diagnosed with a stage T2a melanoma of her back in March 2015 and
underwent a 2 cm wide local excision with negative sentinel lymph
node biopsy. Surveillance imaging and subsequent biopsy in March
2016 revealed metastatic melanoma in the lung, treated with wedge
resection. In February 2017, surveillance MRI detected a subcutaneous
nodule of the posterior trunk; pathology confirmed metastatic
disease. After surgical excision of the nodule, the patient was started
on high-dose ipilimumab in May 2017. While on ipilimumab, the
patient developed new foci of disease with subcutaneous nodules
on the back and later on the abdomen. Both lesions were resected Ipilimumab was discontinued, and she was started on nivolumab in
January 2018. After two months of treatment with nivolumab, the
patient presented to the emergency department with progressive
lower lip ulcerations and gingival erosions associated with burning,
bleeding, and difficulty tolerating oral intake secondary to pain. She
was initially treated with valacyclovir without improvement. Viral
culture was negative for herpes simplex viruses 1&2. She was referred
to dermatology for further evaluation. On physical examination, an
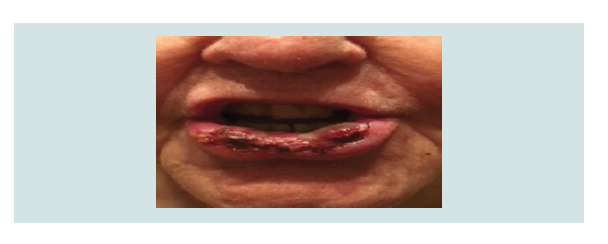
extensive 4 cm heme-crusted tender ulceration was noted on the
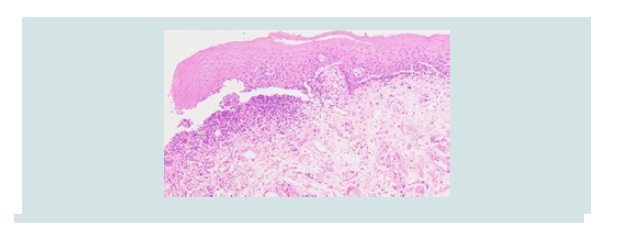
lower vermilion lip (Figure 1). Punch biopsy revealed erosive lichen
planus (Figure 2). Nivolumab was temporarily held, and the patient
was treated with topical clobetasol 0.05% ointment and bacitracinpolymyxin
ointment twice daily for two weeks followed by topical
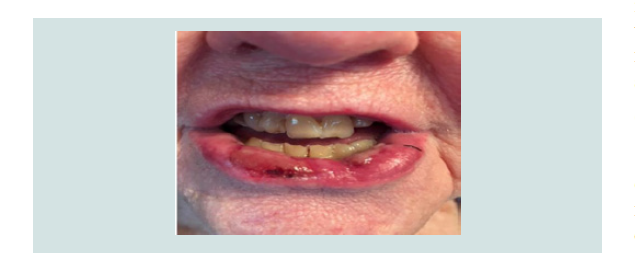
tacrolimus 0.1% ointment twice daily. Significant improvement in
the lesion was noted after one month of treatment (Figure 3). The
patient resumed immunotherapy after 4 weeks, and her oral lichen
planus remained stabilized with topical therapy. She subsequently
developed bullous lesions on her bilateral shins and feet. Pathology
demonstrated a lichenoid process, and direct immunofluorescence
studies were negative for immune deposits.
Case 2: A 70-year-old male with a history of psoriasis was referred to
dermatology after diagnosis of a T4bN1aM0 melanoma of the right
arm, status post wide local excision and axillary sentinel lymph node biopsy
One of four lymph nodes was positive for melanoma, and the
patient underwent a complete right axillary lymph node dissection
with no additional positive lymph nodes. PET/CT and MRI scans did
not show evidence of metastatic disease. The patient was enrolled in a
clinical trial of adjuvant immunotherapy randomized to nivolumab or
combination ipilimumab/nivolumab in February 2018. Two months
after starting treatment, the patient presented to his oncologist with
a lip ulceration, which was initially treated with topical acyclovir
without improvement. Evaluation by a dermatologist revealed 2
cm heme-crusted erosion on the lower vermilion lip. Punch biopsy
was consistent with oral lichen planus. The patient was started on
topical clobetasol 0.05% ointment twice daily for one week followed
by alternating topical tacrolimus 0.1% ointment twice daily Monday-
Thursday with clobetasol 0.05% ointment twice daily Friday-
Sunday, with notable improvement of the lesion within 3-4 weeks.
Immunotherapy was continued while his oral lichen planus was
treated.
Figure 2: Case 1- hematoxylin and eosin stain showing a lichenoid infiltrate with squamatization of the basal layer, consistent with lichen planus.
Discussion
Lichen planus is a CD8+ T-cell mediated autoimmune process
targeting basal keratinocytes causing apoptosis of epithelial cells and
resultant erosions and ulceration of mucocutaneous sites. The precise
mechanism of drug-induced lichenoid reactions is not completely
understood, but likely involves breaking the normal tolerance of
CD8+ lymphocytes for the epithelium. One signaling pathway which
may induce tolerance for the epithelium is through the interaction
of PD-1 with ligand B7-H1 [4]. Blockade of this interaction by
pembrolizumab and nivolumab likely tips the delicate balance of
tolerance allowing CD8+ lymphocytes to react against epithelial
cells. A number of other medications have been also associated
with oral lichenoid reactions, including angiotensin-converting
enzyme inhibitors, anticonvulsants, antiretrovirals, nonsteroidal
anti-inflammatory drugs, and more recently TNF-alpha and tyrosine
kinase inhibitors, though the pathogenesis is likely different than that
of checkpoint inhibitors [5,6].
Several cutaneous adverse effects of PD-1 inhibitors have been described, however, literature detailing oral mucosal toxicity and
optimal treatment strategies for this toxicity in patients treated with
checkpoint inhibitors for melanoma is more limited. Of lichenoid
eruptions [7,8], one study found mucosal involvement in only 1 of 14
patients [8]. Here we present two patients with advanced melanoma
who developed oral lichen planus within two months of treatment
with nivolumab. Both patients initially presented with isolated
oral involvement. In our first patient, the extensive nature of and
symptoms from her lichen planus led to temporary discontinuation
of nivolumab. The second patient did not require holding of
immunotherapy. Both patients improved with very high potency
topical steroids and topical calcineurin inhibitors, allowing the
first patient to resume nivolumab. Oral glucocorticoids or systemic
steroid-sparing immunosuppressive agents were not used.
Conclusion
Our cases support considering oral lichen planus as a notable
cutaneous adverse event in patients receiving PD-1 inhibitor therapy
for the treatment of melanoma. Recognition of erosive lichen planus
early as an adverse event by both oncologists and dermatologists may
potentially avoid disruptions in immunotherapy with appropriate
treatment. Also, this experience suggests that topical therapy
alone either with high potency topical steroids or in combination
with tacrolimus ointment can provide a sustained response while
immunotherapy is continued. Of note, both patients had comorbidities
(DLBCL and psoriasis) suggestive of baseline immune dysregulation.
Future research investigating specific cutaneous adverse effects of
checkpoint inhibitors can yield more definitive answers about their
association with development of other immune-mediated adverse
reactions and implications for tumor response to therapy.