Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Efficacy and Safety of Low-Dose Aspirin for Venous Ulcers: Randomized Clinical Trial
Sanchez-Dipasupil E*, Gulmatico-Flores Z and Lopez-Villafuerte L
Department of Dermatology, Jose R. Reyes Memorial Medical Center,
Philippines
*Address for Correspondence: Sanchez-Dipasupil E, Department of Dermatology, Jose R. Reyes Memorial Medical Center, Philippines, Phone: +639178777978; E-mail: edessahmd@gmail.com
Submission: 16 November, 2019
Accepted: 20 December, 2019
Published: 23 December, 2019
Copyright: © 2019 Sanchez-Dipasupil E, et al. This is an open access
article distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Background: Venous leg ulcers are chronic relapsing wounds which impair
quality of life and has significant health economic burden. Many venous leg ulcers
take over 6 months to heal and a quarter will fail to heal completely. Current
standard treatments include compression therapy, bandages and Pentoxifylline.
Few published trials in United Kingdom and Spain demonstrated that Aspirin
may hasten healing of VLUs.
Methods: A prospective, randomized, double-blind, placebo-controlled trial
was conducted to provide evidence regarding the efficacy and safety of Aspirin in
addition to standard care. Patients aged 18 to 75 with at least one chronic venous
leg ulcer were recruited. Both the treatment and placebo groups were instructed
to take 2 tablets of 80 mg Aspirin once daily after breakfast for a maximum of
12 weeks or less in case of complete ulcer healing. Eligible patients were stratified
according to size (≤ 5 cm2 and > 5 cm2). The primary outcome measure was the
proportion of patients with wound closure described as eschar formation over the
entire surface. Safety outcomes were assessed in all participants.
Results: A total of 34 patients were enrolled in the study. In the aspirin group,
33.33% of patients had complete ulcer closure compared with 0% in the control
group (RR 0.6667, 95% CI 0.4661 to 0.9535, P = 0.0264). Mean healed area
of ulcer in the aspirin group was 36.19 cm2 compared to 21.39cm2 in the control
group. Healing time for ulcers in the aspirin group was at 6 ± 2.19 weeks.
Conclusion: This study demonstrated that Aspirin 160mg has a favorable
effect and can be given as an adjunct to standard wound care and compression in
the management of venous ulcers. Proper selection of patients will prevent adverse
effects and improve healing rate and time.
Introduction
Venous leg ulcers are a type of chronic wound aff ecting up to
1% of adults in developed countries at some point during their lives
[1]. It accounts for 80 percent of lower extremity ulcerations [2]. The venous leg ulcer is an age related disease in the elderly population,
especially women. Th e primary risk factors are older age, obesity,
previous leg injuries, deep venous thrombosis, and phlebitis [3].
Dormandy, in reviewing the pathophysiology of venous leg ulcers,
stated that the currently favored hypothesis for the link between
the increased venous pressure of chronic venous insuffi ciency and
venous ulcer is based on the intermittent inappropriate activation
of white blood cells [4]. Th e damage initiated by the oxidative burst
of the leukocytes leads to endothelial dysfunction, interstitial edema,
microthrombi, and long-term microcirculatory damage, including
decreased capillary density. Th e net result is impairment of the
potential for healing and hence ulcer formation [5].
Whereas the venous leg ulcer is usually originated by external
trauma, the course is oft en chronic and/or relapsing [3]. Many venous
leg ulcers take over 6 months to heal; one large study demonstrated a median time to ulcer healing of 99 days with 2-layer compression
therapy. In addition, more than a quarter fail to heal completely and
the 12-month recurrence rate of healed venous leg ulcers may be up
to 28%. Patients with longstanding, large ulcers, or who have a prior
history of ulceration, are particularly resistant to healing [6].
Th ere are various treatment options for venous ulcers. Th ese include
conservative management, mechanical treatment, medications, and
surgical options. Evidence rating a key recommendation includes
compression therapy, bandages and Pentoxifylline [2]. Compression
assists by reducing venous hypertension, enhancing venous return
and reducing peripheral edema. However, studies show that it only
has moderate eff ects on healing, with up to 50% of venous leg ulcers
unhealed aft er two years of compression. Non-adherence may be the
principal cause of these poor results, but presence of inflammation in
people with chronic venous insufficiency may be another factor, so a
treatment that suppresses inflammation and reduces the frequency of
ulcer recurrence would be an invaluable intervention to complement
compression treatments [7].
Aspirin is a cyclooxygenase inhibitor that irreversibly reduces
prostaglandin-2 and thromboxane A2, which are involved in
infl ammation and platelet aggregation. It is inexpensive, widely used
and readily available. Th e mechanism by which aspirin may hasten
healing of VLUs is unclear but may be associated with a reduction of
infl ammation, or its eff ect on the microvascular circulation, including
platelet activation. In one study investigating the haemostatic eff ects
of aspirin in patients with VLU, the investigators demonstrated that
participants were found to have increased levels of fibrinogen and
shortened coagulation rate, when compared to age-matched and sexmatched
controls and that treatment with aspirin caused prolongation
of the coagulation rate, which increased the rate of ulcer healing [6].
Venous leg ulcers impair Quality of Life (QoL); they are open
wounds, which can be large, are oft en painful, frequently become
infected and leak exudate. It has signifi cant health economic burden.
Th ere is, therefore, an unmet need for a more cost-effective and
clinically eff ective treatment for VLUs.
Methodology
Patients and research design:
Th is is a prospective, randomized, double-blind, placebocontrolled
trial to provide evidence regarding the efficacy and safety of
aspirin, at a dose of 160 mg once daily, in addition to standard care in
patients with chronic venous leg ulcers conducted at the Jose R. Reyes
Memorial Medical Center Dermatology Outpatient Department.Patients recruited included male and female patients aged 18 to
75 with at least one chronic venous leg ulcer confirmed by duplex
scan to be venous in origin. Patients included should be willing to
have elastic compression therapy. The ulcers had to be open for at
least 6 weeks.
Th e exclusion criteria included those who are unable or unwilling
to provide consent, patients with diabetes mellitus and/or peripheral
arterial occlusive disease, those with leg ulcers of non-venous
etiology, on regular concomitant aspirin, with previous intolerance
or contraindication to aspirin use, taking prohibited medications
such as, probenecid. Oral anticoagulants including coumarins
and phenindione, dabigatran, heparin, clopidogrel, dipyridamole,
sulfi npyrazone and iloprost, with history of peptic ulcer / dyspepsia,
with known lactose intolerance, pregnant or lactating women,
patients
with infection of deeper skin structures or with intensive
involvement requiring systemic antibiotics, those who have been
given oral or topical antibiotics within 2 weeks and/or other reasons
that excludes them from participating within this trial made by the
investigator’s clinical judgment.
Approval from the Institutional Review Board of the hospital was
obtained prior to commencement of the trial. Eligible patients were
asked to sign a written informed consent prior to their inclusion to
the study. All patients were fully informed about the nature of the
research study and the chances of being randomized to either the trial
drug (aspirin) or placebo. Eligible patients were stratifi ed according
to size (≤ 5 cm2 and > 5 cm2). Th is size stratifi cation was used because venous ulcers > 5cm2 have been shown to have slower closure rates
than smaller wounds.
Materials:
Th e aspirin (80 mg) and placebo tablets of the same size, color
and shape were placed in identical bottles. Th e bottles were labelled
as A or B by a co-investigator.Randomization, treatment allocation, and blinding
A computer-generated random sequence was used for allocation
of recruited subjects. Subjects and investigator were blinded until
aft er the completion of the study.
Study intervention:
All participants underwent standardized, local management of
ulcers which includes wound cleansing with saline solution, saline
presses twice daily, compression therapy and leg elevation.Patients under the treatment and placebo groups were instructed
to take 2 tablets of given medication once daily aft er breakfast for a
maximum of 12 weeks or less in case of complete ulcer healing. All
participants were advised to avoid consumption of acidic foods such
as alcoholic beverages and coffee.
Patients were also given a special instruction to immediately
discontinue the medication and inform the investigator if they
experience signs of gastric irritation.
Clinical assessment:
All patients were evaluated by the same dermatologist at baseline
and every 2 weeks until closure or a maximum of 12 weeks. Digital
photographs and ulcer surface area were obtained every 2 weeks.Th e primary outcome measure of the study was the proportion
of patients with complete healing in each group described as eschar
formation over the entire wound surface. Secondary outcome
parameters included time to complete healing, percentage of surface
area healed and the incidence of adverse events.
Th e surface area of the ulcer was measured using manual
planimetry. Number of grids found within the traced circumference
over an acetate film was counted and multiplied by the area in square
centimeters. All partial grids of more than 50% were included.
Baseline CBC, creatinine, SGPT, SGOT, bleeding time, clotting time
and urinalysis were requested.
Stopping guidelines:
All patients who develop unacceptable treatment toxicity which,
in the investigator’s opinion, is attributable to the intervention were
withdrawn from the study but follow-up was continued to enable an
intention-to-treat analysis. Th e side effects associated with aspirin
include, but are not limited to, gastrointestinal hemorrhage and
gastrointestinal disturbance including dyspepsia, and ulceration.
Those who had worsening of skin lesions were withdrawn and
treated appropriately. Those who did not comply with the single daily
dosing of the tablets, or those who used other medications were also
withdrawn.Dropouts were defi ned as those who did not follow up within 2
weeks and whose outcome were unknown by the end of the study
period.
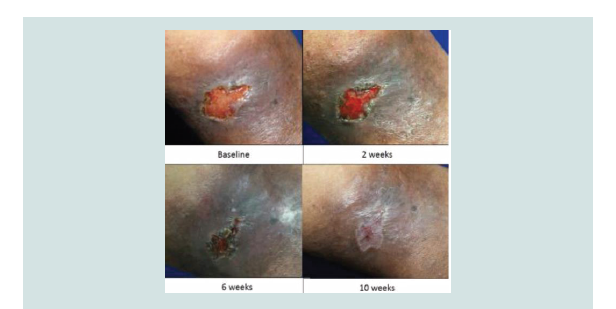
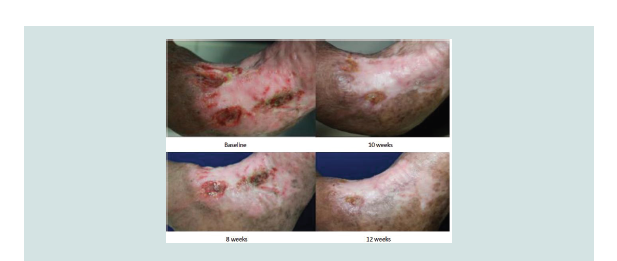
Figure 2: Wound healing of treatment group at baseline, 2 weeks, 6 weeks and complete closure at 10 weeks
Results
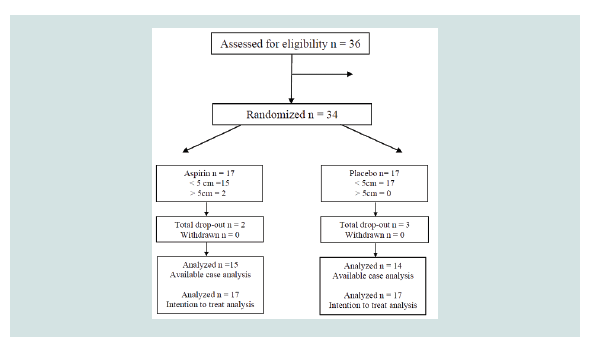
Thirty-six patients were evaluated for eligibility between January
2017 and September 2017. Two patients diagnosed with diabetes
mellitus were excluded. A total of 34 patients were enrolled in the
study, with 17 patients assigned to each arm. Aspirin group had 2
drop outs owing to nonattendance at scheduled visits (Figure 1).
Majority of the subjects were females (61%) with 64.71% (11/17)
from the treatment group and 58.82% (10/17) from the placebo group.
Mean age for both groups was 53.15, 53.71 years for the treatment
group while 52.59 years for the control group. Th e mean duration
of venous leg ulcer for the treatment group was 22 months and 24
months for the control group. Mean size of the ulcer on baseline
was 2.35 cm2, measuring 2.51 cm2 for the treatment group and 2.18
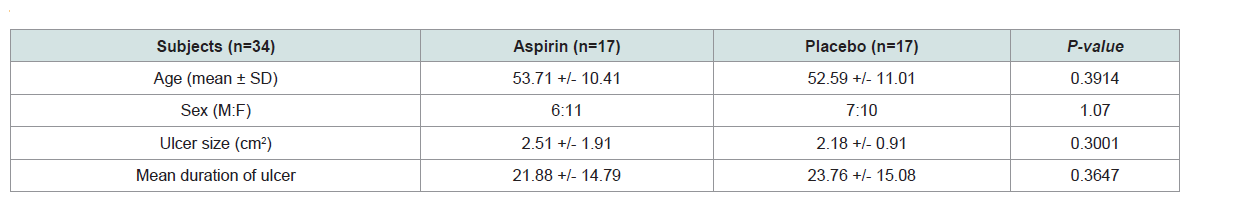
cm2 for the placebo group. Th e baseline characteristics of the study
population are summarized in (Table 1). T-test and chi-square tests
were done. No statistically significant differences were noted between
the two groups based on age, sex, duration of venous disease and
baseline ulcer surface area.
Clinical Effects:
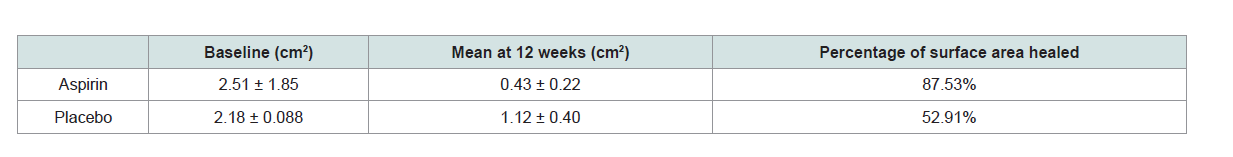
In the aspirin group, 33.33% of patients had complete ulcer
closure compared with 0% in the control group (RR 0.6667, 95% CI
0.4661 to 0.9535, P = 0.0264) (Table2 and 3). All of these ulcers measured
≤ 5 cm2. Th ere were only two subjects with ulcers ≥ 5 cm2 enrolled
under the treatment group and none from the control group. Neither
of these showed complete healing. Mean healed area ofulcer in the aspirin group was 36.19 cm2 compared to 21.39 cm2
in the control group. Healing time for ulcers in the aspirin group were
at 6 ± 2.19 weeks.
T-test was used to determine if there was a difference in the
percentage of ulcer surface area aft er treatment between the
treatment and control groups. With a p value of 0.0064, the ulcer size
of the treatment group significantly decreased in comparison with the
placebo.
Relative risk reduction computation revealed that aspirin, in
addition to standard wound care and compression, will improve
venous ulcers 33% more. ARR was 0.33, favouring aspirin with 33%
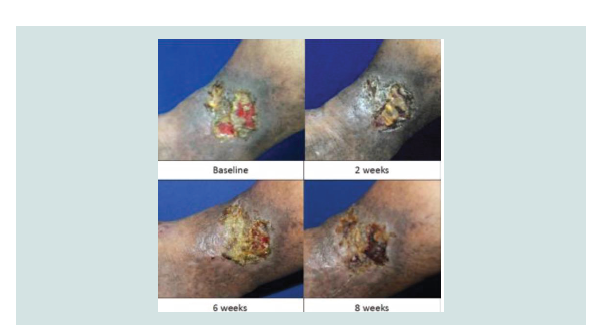
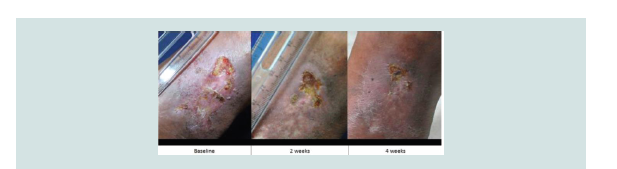
chance of healing. NNT analysis revealed that three patients were required to be treated with aspirin to demonstrate complete ulcer closure. No adverse effects were noted in both groups (Figure 2-5).
Discussion
Th is study demonstrated that Aspirin has a favorable effect in the
management of venous ulcers when used with compression stockings
based on the higher proportion of healed ulcers, faster healing time
and higher percentage of healed surface area. Th ere were no adverse
effects reported.
It was shown that administration of low dose aspirin is 33%
more beneficial than the standard care alone and increases the
chances of healing also by 33%. Time to healing was faster, with 6
weeks compared to 99 days median time of ulcer healing with 2-layer
compression therapy [8-10]. Although the difference was significant, it
is important to note that those patients who demonstrated complete
healing had ulcers less than 5 cm2 and duration of 12 months
In both groups, better healing outcomes were seen in patients who
had ulcers of shorter duration and smaller surface area. These results
are in accordance with studies that investigated the associations of
different risk factors and complete ulcer healing, which determined
that longer ulcer duration and larger baseline surface areas were poor
prognosticators of healing, and that age and sex did not seem to affect
the outcome.
Result of this study was comparable with the two published
clinical trials done in United Kingdom and Spain. Limitation of this
study is the small sample size, short observation period, and failure to
investigate recurrence.
Th ere were no adverse effects documented in this study. However,
complete evaluation of patients prior to administration of Aspirin is
prudent. Lanas et al. did a literature review to construct risk-ratio
estimations and determined incidences of cardiovascular and upper
gastrointestinal complications according to the presence of different
risk factors. Based on the results reported by Hernandez-Diaz and
Garcia-Rodriguez, the pooled relative risk of upper GI bleeding was 2.0 for LDA, considered as doses ≤ 325 mg/day. Major risk factors for
the development of upper GI bleeding are: age, male gender, history
of peptic ulcer and concomitant use of NSAIDs, anticoagulants, or
clopidogrel. They presented clinical variables with corresponding
relative risk ratio estimators. Of note, age 15-49 has a RR of 1.0 and
co-therapy with proton pump inhibitor has an RR of 0.4, decreasing
the risk of bleeding by 60% [11-13].
Conclusion:
Aspirin 160 mg daily can be given as an adjunct to standard
wound care and compression in the management of venous ulcers.
Proper selection of patients will prevent adverse eff ects and improve
healing rate and time.