Journal of Clinical and Investigative Dermatology
Download PDF
Case Report
Merkel Cell Carcinoma in a 65 year-old Filipino: A Case Report
Calderon JD1* and Abad-Casintahan F2
1Department of Dermatology, Calderon Skin Clinic, Philippines
2Department of Dermatology, Jose R. Reyes Memorial Medical Center,
Philippines
*Address for Correspondence: Calderon JD, Department of Dermatology, Calderon Skin Clinic,General Santos City, Philippines, Tel: +639177140531; E-mail: justinedcalderon@gmail.com
Submission: 07 January, 2020
Accepted: 10 February, 2020
Published: 12 February, 2020
Copyright: © 2019 Calderon JD, et al. This is an open access article
distributed under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original work is properly cited.
Abstract
Merkel Cell Carcinoma (MCC) is a rare aggressive tumor known to be
metastatic to the lymph nodes with a poor prognosis. It is commonly characterized
as a painless rapidly enlarging papule or nodule, which may be skin-colored,
erythematous to violaceous. Being twice as uncommon as melanoma, it is seen
among Caucasian elderly males. A case of a 65-year-old male, known hypertensive
with chronic kidney disease, with a sudden enlargement of a painless nodule (2 x
2 x 1 cm) over the left temple is presented in this report. Initial histopathologic
finding was lymphoma, however due to its rapid enlargement further work-up
with imnunohistochemical stains done confirmed the diagnosis of MCC. The
development of MCC in a Filipino is rare and its detection requires a high index
of suspicion. Upon referral to plastic surgery and radiation oncology, wide local
excision and adjuvant radiotherapy were advised respectively. A wide local excision
with margin control and subsequent skin-grafting was done. Thirty-three sessions
of radiotherapy was advised however patient refused the suggested adjuvant
treatment. Absence of nodal involvement and metastasis were documented with
CT scan of the head, neck and abdomen showing no signs of adenopathy
Introduction
Merkel Cell Carcinoma (MCC) is an uncommon, highly
aggressive cutaneous neoplasm. The tumor is most oft en
asymptomatic but rapidly enlarging which may have tendency for
regional and distant nodal involvement. It is commonly seen among
elderly Caucasians with immune suppression and known exposure
to ultraviolet radiation. Th ere is another etiology where in the
Merkel Cell Polyomavirus (MCV) was shown to infect 80% of these
carcinomas. Tumor clonal expansion of tumor cells was said to be
preceded by MCV infection and integration. With this, lesions are
most commonly seen in the head and neck and sun exposed areas
of the extremities. A reported annual incidence of MCC in the US
was noted to have increased for 25 years from 0.15 per 100,000 to
0.44 per 100,000 [1] . Being a rare but lethal neoplasm, MCC is usually
mistaken for a diff erent cutaneous malignancy until proper work-up
has been made [2]. MCC requires aggressive management as it has
a high rate of recurrence and oft en has spread to the regional lymph
nodes at the time of diagnosis. A case of a 65-year-old male, known
hyptertensive with chronic kidney disease for 3 years, with a sudden
enlargement of a painless nodule over the left temple is presented
in this report. Initial histopathologic finding was lymphoma where
immunohistochemistry done showed findings consistent with MCC.
Treatment with wide local excision with margin control with postexcision
skin grafting was done. Subsequent CT scan of the head,
neck and abdomen were also done to note for regional and distant
involvement and metastasis.
Case Report
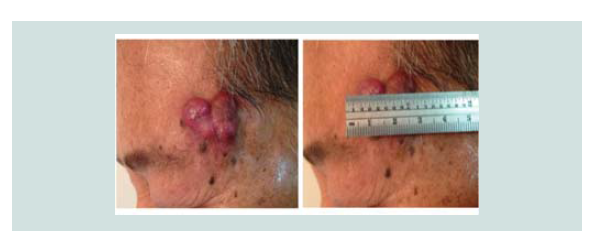
This is a case of a 65-year old male, who presented at our
institution due to a solitary erythematous annular plaque over the left temple. About two months prior to consult, patient noted a painless, non-pruritic erythematous plaque over his left temple. Consult
done at our institution where a 4 mm punch biopsy was done.
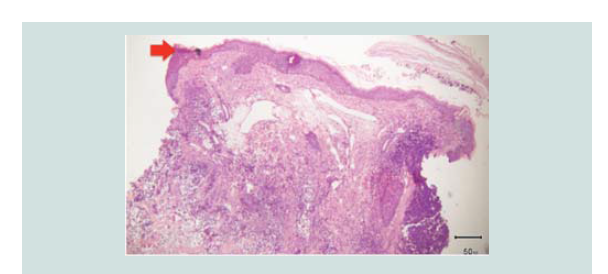
Histopathologic findings showed a flattened epidermis with diffuse
infiltrates from the dermis extending to the subcutaneous layer.
This was initially diagnosed and read as lymphoma. Two weeks later, still
asymptomatic, a sudden enlarged erythematous nodule (2 x 2 x 1
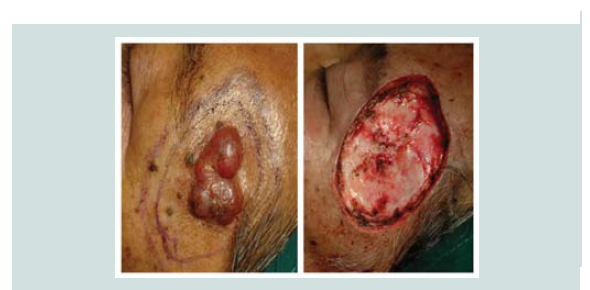
cm) with telangiectasia was noted over the biopsy site (Figure 1). A
repeat biopsy was done and review of the previous biopsy was done.
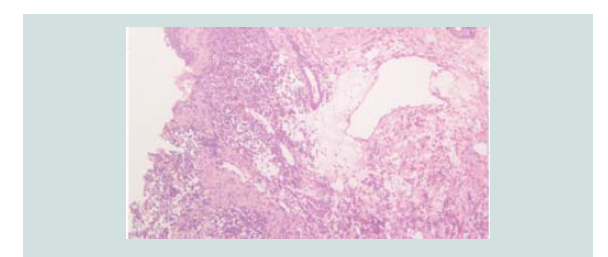
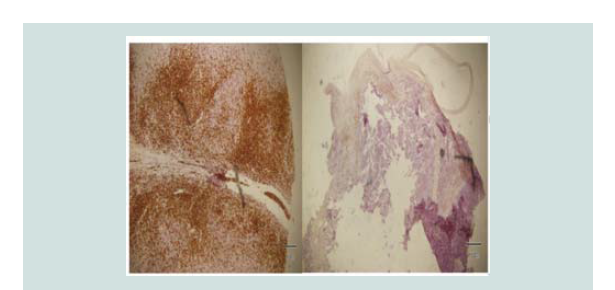
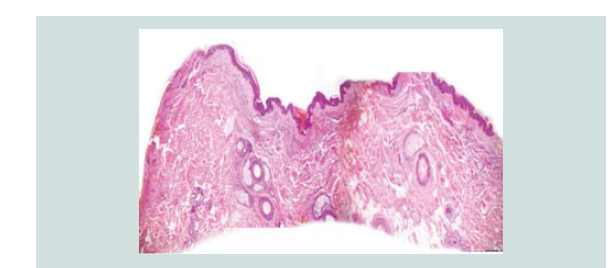
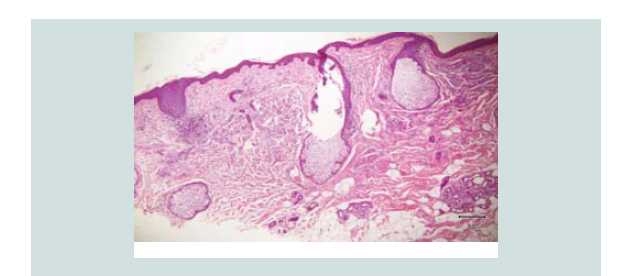
Histopathologic reading showed a dome shaped asymmetrical nodule
with flattened epidermis. In the dermis are nodules of different
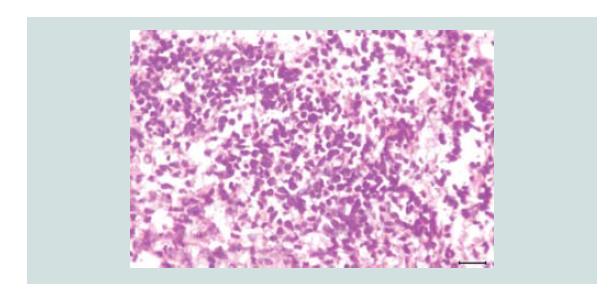
sizes and shapes consisting of neoplastic cells of round blue cells
with scanty cytoplasm and irregular nuclei closely spaced in sheets
and trabecular pattern. Nuclear chromatin is dense and uniformely
figures and nuclear fragments are seen per power field (Figures 2-4 and Figure 6).
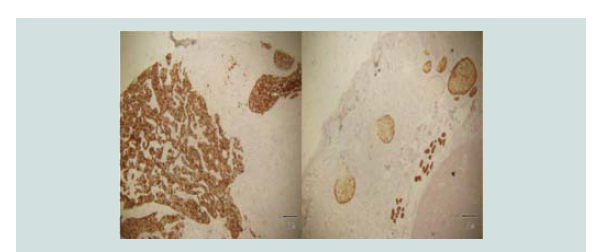
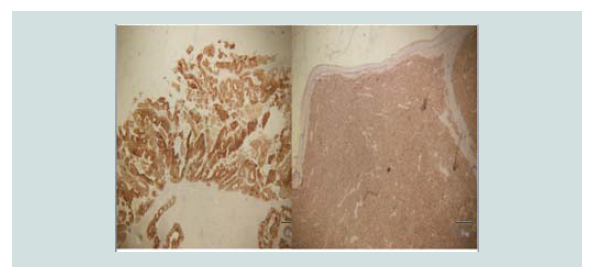
Immunohistochemical stains were done which showed negative CD
3, CD 20, CK 7 and positive CK 20 (Figure 5-8). These findings were
noted to be consistent with merkel cell carcinoma.
Review of systems showed anorexia. On physical examination,
blood pressure was elevated at 140/80 mmHg. The rest of the systemic
findings were unremarkable. The patient has comorbidities of
hypertension, chronic kidney disease and benign prostatic hyperplasia
for 3 years where patient is compliant with his maintenance
medications. He previously had hemorrhoidectomy about 10 years
prior and he just previously had an arteriovenous fistula graft creation
over his right arm. However, no hemodialysis has yet been initiated.
Patient has no previous malignancies with no known family history as well. Th e patient is a retired bank teller, with occasional intake of alcoholic beverage and 24.5 pack years of cigarette smoking. Upon
initial laboratory work-up, complete blood count showed anemia, proteinuria on urinalysis and elevated serum creatinine, which may be attributed to the patient’s kidney disease
Immediately aft er release of histopathologic results, patient was
referred to plastic surgery and radiation oncology. The plan was to do wide local excision and 33 sessions of post-excision adjuvant radiotherapy. A wide local excision with adequate margin control was
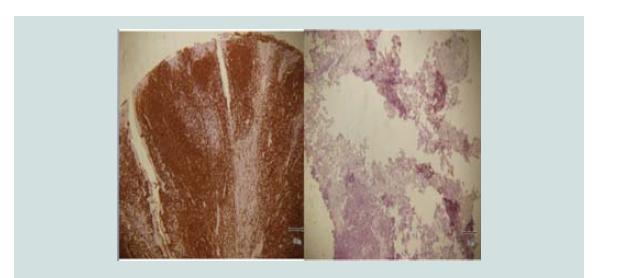
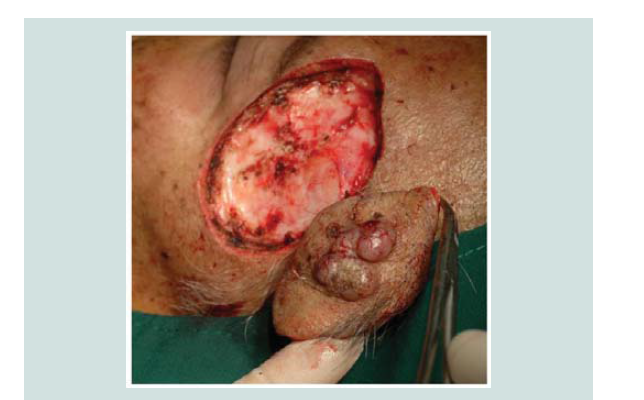
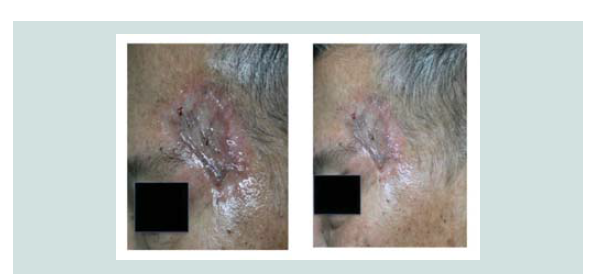
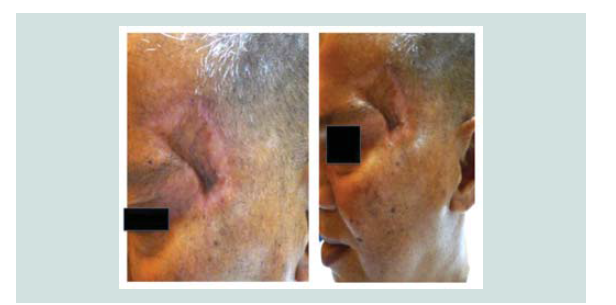
carried out. Review of margins histopathologically showed negative for neoplastic cells (Figure 9-12). A skin graft taken from the patient’s anterior thigh was placed over the excised area. Good wound healing was noted over the graft ed site one month after (Figure 13).Subsequently CT scan of the head, neck and abdomen was requested which also showed negative lymph node involvement or any sign of metastasis. With the result, patient opted to defer the contemplated
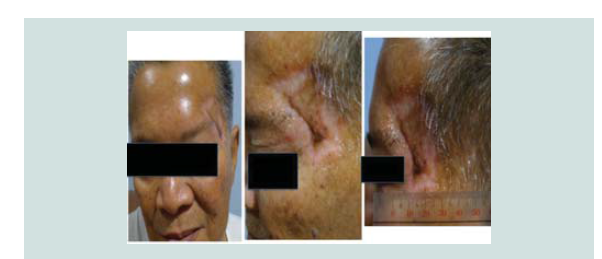
radiotherapy. Seven months aft er the excision, patient still claims to have good wound healing of the graft with clinically no signs of recurrence (Figure 14-16).
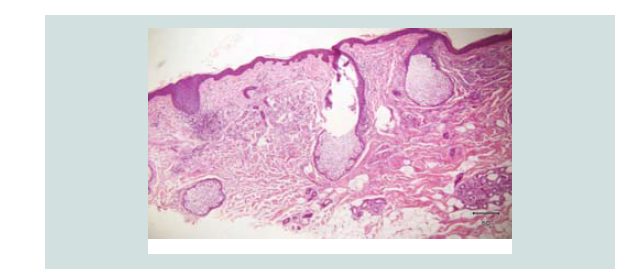
Figure 3: Closer magnification showing nodules of different sizes and shapes. It consists of neoplastic cells of round blue cells with scanty cytoplasm and irregular closely spaced sheets and trabecular pattern.
Figure 4: Aggregates of atypical cells with numerous mitotic figures. These atypical cells consist of atypical nuclei with granular nucleoplasm. Nuclear chromatin is dense and uniform figures of nuclear fragments are also seen
Discussion
Being located in the stratum basale of the epidermis, merkel cells
appear as clear cells that maybe round to oval, elongated or flattened on
light microscopy. It is known to function as a receptor of mechanical
stimuli, specifically sense of touch and hair movement. The origin of
merkel cells has always been of debate. In one hypothesis, it has been
said that similar to the melanocyte, the merkel cell has migrated from
the neural crest. This is based on the association of Schwann cells to
the developing fetus. In an alternate hypothesis, merkel cells were
said to originate from the epidermis as modified keratinocytes. To
support this, desmosomes with keratinocytes within merkel cells has
been documented [3]
The first case of Merkel Cell Carcinoma (MCC) has been described
by Toker in 1972 and was previously termed trabecular carcinoma
[1,2]. Also known as primary neuroendocrine carcinoma of the skin,
primary small cell carcinoma of the skin and cutaneous apudoma,
merkel cell carcinoma is a malignant proliferation of anaplastic cells
associated with a high recurrence and poor prognosis [4]. In the US, its
annual incidence has increased from 0.15 cases per 100,000 to 0.44 per
100,000 in the last 25 years. In the Netherlands, it constituted 0.7% of
all non-basal cell carcinoma skin cancer. Th e European standardized
rate on the other hand was 0.3 per 100,000 person-years from 2001 to
2005 [1]. In Asia, much fewer cases have been documented to date. In
a study done in Mainland China, only 22 cases were seen from 1970
to 2009. In the country, a case report done in this institution has been
documented in 2009 where a patient developed MCC over the gluteal
region. MCC is usually seen among elderly Caucasian males with
immune suppression. Th e most common sites affected are the head
and neck with also a predilection for sun-exposed skin. The etiology which also showed negative lymph node involvement or any sign of metastasis. With the result, patient opted to defer the contemplated
radiotherapy. Seven months aft er the excision, patient still claims of MCC has yet to be determined although multifactorial associations have been determined. Ultraviolet B (UVB) radiation exposure has
been noted to correlate with the disease development where people
living in Hawaii, where UVB index is highest, have shown the highest
incidence of MCC. Another factor is immune suppression and MCC
development. Cases have been reported in patients with autoimmune
disease also aft er solid-organ transplantation, like the kidney, liver
and heart. Patients with acquired immunodefi ciency syndrome also
have an increased risk [1]. Recently Houben et al. have discovered a
possible viral etiology that may contribute to the development of MCC
[5]. A polyomavirus, now referred as the Merkel cell polyomavirus
was shown to infect 80% of these carcinomas. They found viral
DNA integration within the tumor genome in a clonal pattern. It
was suggested that MCV infection and integration preceded clonal
expansion of tumor cells [4,6,7]
MCC typically presents as a painless, pink-red to violaceous, firm
dome-shaped nodule with rapid enlargement. Its surface may be
shiny or may show telangiectasia. To summarize the clinical features
most commonly associated with primary MCC the acronym AEIOU
was developed: asymptomatic or nontender, expanding rapidly,
immune suppressed, older than 50 years, ultraviolet-exposed fair skin
[8]. Common differential diagnoses include basal cell carcinoma,
squamous cell carcinoma, amelanotic melanoma and adnexal tumor.
Because of its violaceous to hemorrhagic appearance, the differentials
may also include pyogenic granuloma, abscess, angiosarcoma and
lymphoma. Histopathologically, the tumor appears as a poorly
defined dermal mass, with frequent infiltration to the subcutaneous
fat, fascia and muscle. The edge of the tumor shows a trabecular
infiltrating pattern and is composed of monotonously uniform, small
round to oval cells that are about two times larger than lymphocytes.
The nuclei appear finely granular dispersed chromatin in a majority
of tumors. Mitotic figures are numerous usually numbering to
about 5-10 per high power field. Nucleoli are usually not seen or not
prominent. With immunohistochemical stains to aid in the diagnosis,
there is a characteristic perinuclear globule upon staining for low molecular-
weight Cytokeratins (CK) such as CK 20, CK 5/6 and
CK 7. Reportedly CK 20 is the most sensitive and specific marker
for detecting micrometastases in sentinel lymph node biopsies.
Staining for various neuroendocrine markers can be added where
MCC will show positive staining for chromogranin, synaptophysin,
somatostatin, calcitonin and vasoactive intestinal peptide. With the
recognition of Merkel cell polyomavirus, immunohistochemical and
molecular biologic methods can provide additional tools for diagnosis.
Electron microscopic studies of carcinoid, neuroendocrine, and
primary Merkel cell carcinomas show “neurosecretory” membranebound,
dense-core granules measuring 100 to 250 nm in diameter
[4-6,]8
The American Joint Committee on Cancer staging system is
similar to the 4-tier system from the Memorial Sloan-Kettering Cancer Center. The said MCC staging system is based on tumor size, lymphatic spread, and distant metastasis. The 4-tier staging includes
the following: stage I for primary lesions <2 cm, stage II for primary
lesions >2 cm, stage III for positive lymph nodes and stage IV for
those with distant metastasis. The staging is used to determine the
appropriate treatment and to predict disease prognosis. A 10-year
relative survival rate is correlated to the tumor size and is 61% for
patients with tumors < 2 cm and about 40% for patients with tumors
>2 cm. A recurrence rate of 40% has been reported [4,6]
For the treatment, surgery is still the primary approach. In a study
by Fields et al, they have reviewed recurrence aft er complete resection
and the selective use of adjuvant therapy for Stage I through III MCC.
A low recurrence rate in patients with clinically lymph node-negative
MCC can be achieved with adequate surgery and that the selective
use of adjuvant radiotherapy is recommended for high-risk tumors
[9]. In an evidence-based review of the management of primary and
localized MCC by Ellis et al, the mainstay of approach for newly
diagnosed MCC remains surgical. Their current recommendations
are based on the size of the primary tumor and excision with 1cm
margins. Some studies have suggested a 2cm margin. But since most
of the lesions are located in the head and neck, the aesthetic and
functional outcomes of surgery must be considered where a smaller
margin is recommended. The use of Mohs micrographic surgery
is still a relatively new modality and that its benefit over wide local
excision include tissue conservation [10-12]. It has been shown to be
as efficacious as wide excision in treating localized MCC. There have
also been enough data to advocate the use of adjuvant radiotherapy
owing to the high incidence of recurrence and metastasis. Adjuvant
radiotherapy has been of greatest benefit in improving the survival
of cases where tumors were >2 cm. However, there is little evidence
that suggested that the use of radiotherapy in those with histologically
negative margins in reducing local recurrence rates. When used as
monotherapy, radiotherapy is only used when in inoperable cases.
Chemotherapy is only reserved for systemic disease and with very
limited success, no chemotherapy protocol has yet been shown to
increase survival [8,13,14].
Conclusion
Merkel cell carcinoma presenting in an elderly Filipino male
over a period of less than 3 months is relatively rare. Early diagnosis
and management is key to the control of the disease as it is a highly
aggressive neoplasm with a propensity for local recurrence. A high
index of suspicion is also key to the early detection of the disease.
One must keep in mind the clinical AEIOU’s of MCC, which include:
asymptomatic or nontender lesion, expanding rapidly, immune
suppression, older than 50 years and ultraviolet light exposure.
Appropriate work-up must be done such as immunohistochemistry
to confirm the diagnosis. For localized MCC, surgical excision is
still the treatment of choice. Additional adjuvant radiotherapy must
be highly considered especially for more advanced stages of the
disease at diagnosis. The use of skin grafting may also be of benefit
in providing tissue conservation. Evaluation for regional and distant
metastasis along with close follow-up must be done accordingly to
improve survival.