Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Topical Application of Curcumin Longa 5% Rhizome Ethyl Acetate Extract in Ointment Form versusMupirocin 2% Ointment in the Treatment of Impetigo and Folliculitis: A Double Blind, Randomized Controlled Trial
Gutierrez EMC* and Gulmatico-Flores Z
Department of Dermatology, Jose R. Reyes Memorial Medical Center, Philippines
*Address for Correspondence:
Gutierrez EMC, Second year resident, Department of Dermatology,
Jose R. Reyes Memorial Medical Center, Philippines; E-mail: emcgutierrez@yahoo.com
Submission: 15 January, 2019
Accepted: 27 February, 2019
Published: 29 February, 2019
Copyright: © 2020 Gutierrez EMC, et al. This is an open access article
distributed under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original work is properly cited.
Abstract
Introduction: Impetigo is a common dermatosis of childhood commonly caused by Staphylococcus aureus and Group A Streptococcus. Antibiotics, topical or systemic,
are the backbones of current treatment of impetigo. The spread of multi-drug resistant pathogens is one of the most serious threats to successful treatment of bacterial
diseases. According to the World Health Organization, the use of medicinal plants is the most common form of traditional medication worldwide. Curcumin longa,
commonly known as yellow ginger or turmeric or luyangdilaw, is a sterile plant from the Zingiberaceae family. Curcumin longa has been shown to possess significant antiinflammatory
and anti-microbial effects.
Objectives: The general objective of this study is to determine the efficacy and safety of Curcumin longa 5% rhizome ethyl acetate extract in ointment versus mupirocin
2% ointment in the clinical improvement or resolution of localized impetigo and/or folliculitis among patients seen in the JRRMMC, Department of Dermatology, and
outpatient department.
Methods: Patients with localized impetigo and/or folliculitis that met the inclusion criteria were recruited. Informed consent obtained, photographs taken at baseline
(week 0). Similar subjects were used to compare both medications Group A (mupirocin 2% ointment) and Group B (Curcumin longa 5% rhizome ethyl acetate extract in
ointment) to assure that we are dealing with the same microorganism. All patients were instructed to clean the lesions using normal saline solution using a gauze or cotton
and to apply the afore mentioned medications using a cotton swab thrice daily for two weeks, or until resolution of lesions are noted. The patients were examined every week
for 2 weeks and the primary investigator assessed if there is a reduction in the number of lesions and evidence of local adverse reaction (erythema, pain, itch, crusting, etc.).
Photographs were taken every visit to assess improvement. Another resident physician in the same department was blinded and was assigned to distribute the ointments, in
identical containers, to the recruited and qualified patients. Safety evaluation consisting of monitoring and recording all spontaneous adverse events were also noted.
Results: Thirty eight patients were included in the study. 135 lesions were treated with Mupirocin, 133 with Curcumin longa. Of the 38 participants, three (7%) were
considered to be dropouts owing to lost to follow up. After two weeks of treatment, 71.05% (27/38) of the mupirocin group and 52.63% (20/38) of the Curcumin longa
group had resolution of the lesions; however, these differences are not statistically significant (p-value = 0.08). Female patients with age groups of 2 to 5 and 6 to 10 were
noted to have the highest improvement both at 13%. Male patients ages 2 to 5 were noted to have the highest improvement at 10%. The mean Skin Infection Rating Scale
(SIRS) after first week for the mupirocin group was 0.93 and 1.46 for the Curcumin longa group, and after second week for the mupirocin group was 0.37 and 0.83 for
the Curcumin longa group which in both cases reflects large improvements relative to baseline (p-values < 0.001), but insignificant differences from each other (p-value =
0.063 and 0.074 respectively). Mupirocin-treated lesions recover significantly quicker than Curcumin longa-treated ones (first week, 72% and 31 %, respectively) with these
proportions being significantly different at a p-value of < 0.032. There were no significant differences in the incidence of adverse effects/withdrawal rates between groups
(p-values of 0.06, 0.28, and 0.09, respectively).
Conclusion: Our results showed that topical application of Curcumin longa 5% rhizome ethyl acetate extract in ointment is of comparable efficacy and safety profile
to mupirocin 2% in ointment in the treatment of localized impetigo and folliculitis. However, mupirocin-treated lesions recover significantly quicker than Curcumin longatreated
ones. Those in the Curcumin longa group also experienced adverse reactions such as pain, erythema, blister formation, exudates, crusting and itch. Generally, the results
implied that at the end of 2 weeks, both treatments had produced similar improvements on lesion counts.
Introduction
Impetigo is a common dermatosis of childhood commonly
caused by Staphylococcus aureus and Group A Streptococcus [1-3]. Impetigo typically presents a transient vesicle or pustule that quickly
evolves into a honey-colored crusted plaque that can enlarge to greater than 2 cm in diameter with surrounding erythema usually on
the face and extremities [1,2]. Antibiotics, topical or systemic, are the backbones of current treatment of impetigo. Recently, estimates of
the global burden of impetigo are 111 to 140 million people especially
from developing countries are affected at any given time [1]. In the
department of dermatology of the Jose R. Reyes Memorial Medical
Center (JRRMMC), there are 175 cases of impetigo in 2014 and 173
in 2015. The spread of multi-drug resistant pathogens is one of the most
serious threats to successful treatment of bacterial diseases [1,3-6].
According to the World Health Organization (WHO), the use of
medicinal plants is the most common form of traditional medication
worldwide. Regulation of herbal medicines is a key means of ensuring
safety, efficacy and quality of herbal medicinal products. WHO has
been receiving an increasing number of requests from governments
for guidance on how to regulate herbal medicines [5]. In 2003, the
Department of Health (DOH) of the Philippines released a list of the
top ten herbal medicinal products that can be safely used [7]. Curcumin longa, commonly known as yellow ginger or turmeric or luyangdilaw, is a sterile plant from the Zingiberaceae family which
originated from India and is now cultivated in other tropical countries
like the Philippines [8-10]. This plant does not produce any seeds and
grows up to 3 to 5 feet tall with dull yellow flowers [8]. Curcumin
longa has been shown to possess significant anti-inflammatory, antioxidant,
anti-carcinogenic, anti-mutagenic, anti-coagulant and antimicrobial
effects [9-18]. Curcumin longa has also been shown to have
significant wound healing properties as it acts on various stages of
wound healing to hasten the process. It also has the ability to enhance
granulation tissue formation, collagen deposition, tissue remodeling
and wound contraction [8,14]. Application of herbal medicinal plants as alternatives to chemical
agents for the treatment of infections has been reported by many
authors, but few researchers focus on these. Therefore, this study aims
to determine the efficacy and safety of the use of topical ointment of
Curcumin longa on impetigo.
We report herein a randomized double blind controlled study
on the efficacy and safety of topical application of Curcumin longa
5% rhizome ethyl acetate extract in ointment form versus mupirocin
2% ointment in the treatment of impetigo and folliculitis in patients
seen at the Department of Dermatology of Jose R. Reyes Memorial
Medical Center.
Methodology
An Institutional Review Board / Institutional Ethics Committee
of the Jose R. Reyes Memorial Medical Center approved the study
prior to its initiation following the guidelines of good clinical practice.
Study design: This study is a double blind, randomized,
controlled trial.
Study setting and duration: The study was conducted at the
Dermatology Outpatient Department (OPD) of Jose R. Reyes
Memorial Medical Center from July 2016 to September 2016.
Inclusion criteria
1. Patients clinically diagnosed with impetigo and/or folliculitis at
the out-patient department clinically
2. Male or Female patient
3. 1 to 30 years old
4. No other constitutional symptoms such as fever, lymphadenopathy
5. Willing to follow-up once a week for 2 weeks.
Exclusion criteria:
1. Patients with known hypersensitivity to any of the test
medications
2. Patients with constitutional or systemic symptoms such as
fever, lymphadenopathy that will need systemic antibiotic treatment
3. Immunocompromised patients
4. Active infected lesions in obvious need of systemic therapy
5. Patients who have been given oral/topical antibiotics within 2
weeks
6. Patients with comorbidities such as diabetes mellitus,
hypertension that in the judgment of the investigator might interfere
with the study.
7. Not willing to follow-up once a week for 2 weeks.Thirty eight patients were recruited in this study. Patients were
recruited during clinic hours in the outpatient department of the
JRRMMC Dermatology and asked to sign a written informed consent.
The patients were interviewed to get a detailed history. They were
also examined by the primary investigator. Information regarding
age, sex, number of lesions and site of involvement were obtained.
No bacteriological examination was done prior to starting the
treatment. The same subject were used to compare both medications
Group A (mupirocin 2% ointment) and Group B (Curcumin longa
5% rhizome ethyl acetate extract in ointment) to assure that we are
dealing with the same microorganism. They were instructed by a
study associate on which lesions will they apply medications A and B.
Areas of application of medications A and B were randomly selected.
The patients were instructed to clean the wound with normal saline
solution, using gauze or cotton. Then the Curcumin longa 5% rhizome
ethyl acetate extract in ointment or mupirocin 2% in ointment was
applied with cotton swab at the center of the lesion thrice a day [2].
All patients were asked to apply the aforementioned medications
for two weeks, or until resolution of lesions are noted. Parents or
guardians were asked to report local side effects (erythema, pruritus,
burning sensation) for this time period.
The patients were examined every week for 2 weeks and the
primary investigator assessed if there is a reduction in the number of
lesions and evidence of local adverse reactions.
The photographs of the lesions were taken at the start of the study
(week 0), every week for 2 weeks (week 1 and 2) to assess effectiveness
and monitor adverse reactions. A digital camera was used and the
photos were taken at the physical examination room of the JRRMMC,
Department of Dermatology, OPD. The primary investigator assessed
the improvement of the lesions of the study participants. Another
resident physician in the same department was blinded and was
assigned to distribute the ointments to the recruited and qualified
patients.
The trial was stopped on patient who experienced irritation
(burning sensation, diffuse erythema, itchiness, pain) or hypersensitity with the topical ointment being used. The patients were considered
as a withdrawal from the study. Any patient who failed to follow up
every week within two weeks and those who failed to comply with the
treatment, or those who used other topical medications other than
the one provided were also included as withdrawals. The results of
those who withdraw from the study were included in the analysis with
an intention to treat principle.
Curcumin longa or yellow ginger were obtained from University
of the Philippines, Los Banos, Laguna that is identified by a botanist.
The Curcumin longa 5% rhizome ethyl acetate extract in ointment
form was prepared by the University of the Philippines-Manila,
College of Pharmacy, Industrial Pharmacy Unit. The mupirocin 2%
ointment purchased from GlaxoSmithkline, a company producing
this medication in the market. Both Curcumin longa 5% rhizome ethyl
acetate and mupirocin 2% in ointment were prepared identically into
a yellow, greasy ointment without any smell and placed in identical
containers.
Outcome measures:
The initial SIRS baseline scores were recorded as a patient-level
average and as such are identical across treatment and control;
subsequent SIRS scores were taken at 1 week and 2 weeks after
treatment onset and recorded as averages of lesions treated with
either mupirocin or Curcumin longa, respectively.Statistical analysis:
Descriptive analysis and summary statistics were performed
using and two-sample t-tests using the Stat Plus. Both per protocol
analysis and intention to treat analysis (primary analysis) were done.
P-values <0.05 were regarded as statistically significant.Results
Patient characteristics:
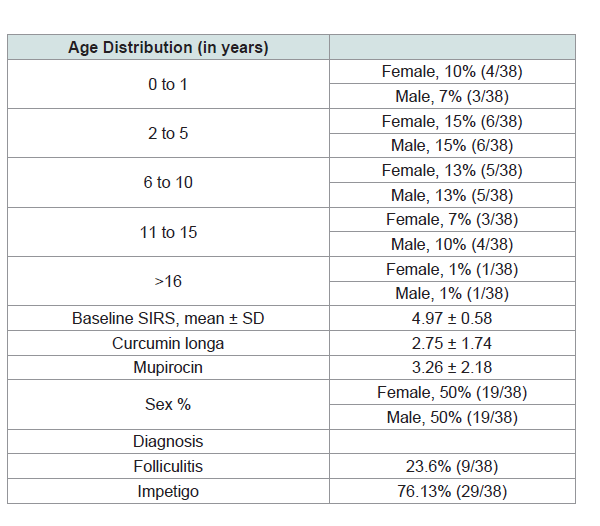
Thirty eight patients were included in the study. 135 lesions were
treated with mupirocin and 133 with Curcumin longa. (Kindly refer
to (Table 1) for the baseline characteristics of the participants) The
biggest components of baseline SIRS were erythema (1.8) and pain/
itch (1.23).
Of the 38 participants, three (7%) were considered to be dropouts
owing to lost to follow up. The dropout rate was in line with the
5-20% expected range in clinical trials. Because patients were each
treated with both mupirocin and Curcumin longa, the dropout rate is
identical between treatment and control.Clinical effects:
4.2.1. Proportions of patients with complete healing: After two
weeks of treatment, 71.05% (27/38) of the mupirocin group and
52.63% (20/38) of the Curcumin longa group had resolution of the
lesions; however, these differences are not statistically significant
(p-value = 0.08) (Table 2).
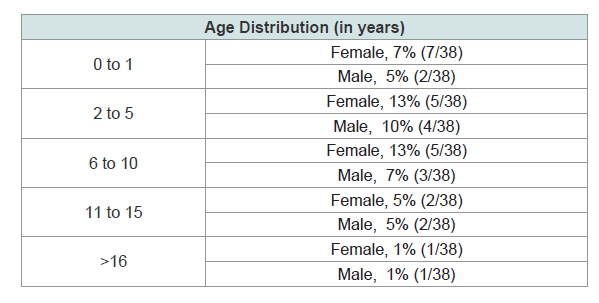
Improvement based on age group and gender distribution.
Female patients with age groups of 2 to 5 and 6 to 10 were noted to
have the highest improvement both at 13%. Male patients ages 2 to 5
were noted to have the highest improvement at 10%.Mean SIRS post-treatment:
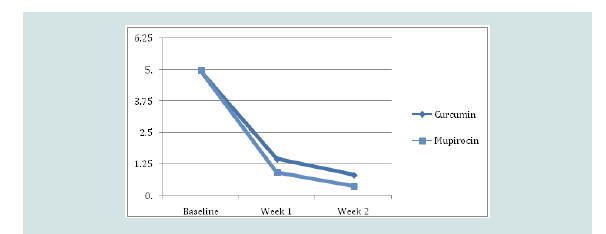
The mean Skin Infection Rating Scale (SIRS) after first week
for the mupirocin group was 0.93 and 1.46 for the Curcumin longa
group, and after second week for the mupirocin group was 0.37 and
0.83 for the Curcumin longa group which in both cases reflects large
improvements relative to baseline (p-values < 0.001), but insignificant
differences from each other (p-value = 0.063 and 0.074 respectively)
(Figure 1).Time to healing:
While the two-week recovery proportions between mupirocin
and Curcumin longa are statistically indistinguishable from each
other, we do see that mupirocin-treated lesions recover significantly
quicker than Curcumin longa-treated ones. After the first week, 72%
of the Mupirocin-treated lesions had recovered as compared to only
31% for Curcumin longa-treated ones, with these proportions being
significantly different at a p-value of < 0.032 (Figure 2).Adverse effects/withdrawal rates:
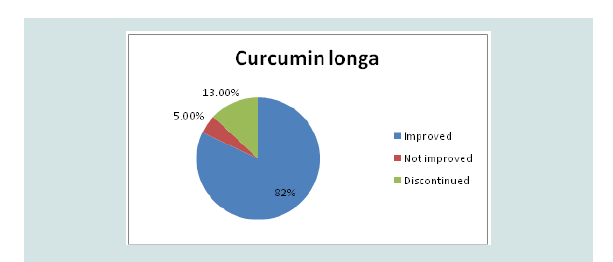
During treatment of Curcumin longa, four patients (10%)
experienced erythema and pain, two (5%) had blister formation, four
(10%) had exudates, thirteen (34%) had crusting and itch. For the
mupirocin ointment group, five (13%) had crusting, eleven (28%) had
erythema and eight (21%) had experienced itch. Five (13%) patients
in the Curcumin longa group prompted to discontinue the treatment
due to adverse effects while two (5%) did not see any improvement.
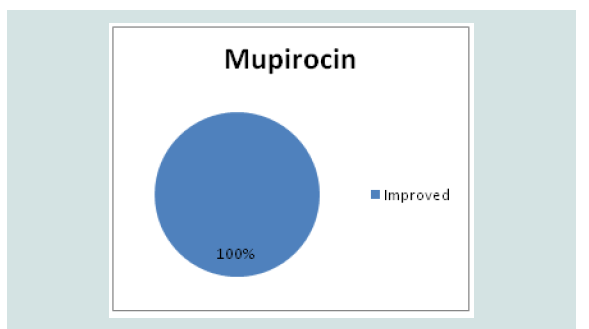
All the patients improved in the mupirocin group (Figure 3).
There were no significant differences in the incidence of adverse
effects/withdrawal rates between groups (p-values of 0.06, 0.28, and
0.09, respectively).Discussion
Impetigo is a highly contagious skin infection that mainly affects
infants and children [1,19]. It is estimated that the global burden for impetigo is 111 to 140 million people especially from developing
countries at any given time [1]. In the department of dermatology of
the Jose R. Reyes Memorial Medical Center (JRRMMC), there are 175
cases of impetigo in 2014 and 173 in 2015.
Impetigo typically presents a transient vesicle or pustule that
quickly evolves into a honey-colored crusted plaque that can enlarge
to greater than 2 cm in diameter with surrounding erythema usually
on the face and extremities [1-4]. Constitutional symptoms are
usually absent but regional lymphadenopathy may be present in up to 90% of patients with prolonged, untreated infection. If untreated,
the lesions may slowly enlarge and involve new sites over several
weeks. In some individuals, impetigo may clear on its own in two
to three weeks, but antibiotics can shorten the course of the disease
and help prevent the spread to others. Treatment of localized, mild
to moderate impetigo with mupirocin ointment or cream, removal
of crusts, and good hygiene is sufficient for resolution [1,20]. Other topical antibiotic agents are: Retapamulin 1% ointment which is
also effective for localized impetigo and secondarily impetiginized
dermatitis as well, although decreased efficacy against MRSA was
noted in some trials [21]; Fusidic acid is an equally effective topical
agent for localized impetigo and has very few adverse effects topically.
Systemic antibiotics may be required in extensive cases [1].
According to the WHO global report on surveillance on
antimicrobial resistance in 2014, it is important to take note that
over the last 30 years, no major new types of antibiotics have been
developed and currently high levels of antibiotic resistance is found
in all regions of the world. Few, if any, of the available treatments
options remain effective for common infections. In South-East
Asia, more than one quarter of Staphylococcus aureus infections are
reported to be Methicillin-Resistant (MRSA), meaning that treatment
with standard antibiotics does not work [5]. The spread of multi-drug
resistant pathogens is one of the most serious threats to successful
treatment of bacterial diseases [1,3,4,6].
According to the World Health Organization, the use of
medicinal plants is the most common form of traditional medication
worldwide. Regulation of herbal medicines is a key means of ensuring
safety, efficacy and quality of herbal medicinal products. WHO has
been receiving an increasing number of requests from governments
for guidance on how to regulate herbal medicine. (WHO Strategy on
Traditional Medicine) [5].
One of the herbal medicines used since the ancient time is the
Curcumin longa commonly known as yellow ginger or luyangdilaw.
Curcumin longa, from the Zingiberaceae family [8]. It is originally
from South India, China and Indonesia but is now currently grown
in most of the tropical countries especially in the South East Asia
including the Philippines [8-10].
The anti-bacterial property of Curcumin longa against a wide
range of gram positive, including Streptococcus and Staphylococcus,
and some gram negative microorganisms has been attributed to the
presence of alkaloid and veleric acid, a byproduct from curcumin
manufacture [9]. Curcumin longa has also been shown to have
significant wound healing properties as it acts on various stages of
wound healing to hasten the process. It also has the ability to enhance
granulation tissue formation, collagen deposition, tissue remodeling
and wound contraction. Most notably, Curcumin was shown to
inhibit the production of tumor necrosis factor alpha (TNF-α) and
interleukin-1 (IL-1), two main cytokines released from monocytes
and macrophages that play important roles in the regulation of
inflammatory responses. Of equal importance is Curcumin’s ability
to inhibit the activity of NF-(κ)B (nuclear factor kappa-light-chain enhancer
of activated B cells), a transcription factor that regulates
many genes implicated in the initiation of inflammatory responses.
For a while, NF-(κ)B has been considered oxidant responsive,
highlighting the correlation between oxidation and inflammation in wound healing. Oxidative stress is a significant factor in the wound healing process and generally inhibits tissue remodeling [14].
In a study done in JRRMMC Department of Dermatology in
2014 by Bernales-Mendoza et al. Streptococcus pyogenes is the most
common microorganism isolated to cause impetigo [22].
In another study done in the same department in 2011 by
Gorkhali et al. an in vitro study using the Curcumin longa extract
showed the following zones of inhibition: 3 to 5 mg/mL concentration
for Staphylococcus aureus and MRSA; 5 mg/mL concentration for
Group A beta hemolytic Streptococcus [23]. An in vivo study was also
done by Gorhkhali et al. using only 4mg/mL of ethyl acetate extract
of Curcumin longa ointment there was only a clinical cure rate in
50% of the patient and they concluded that the use of 4 mg/mL of
ethyl acetate extract of Curcumin longa ointment is not as effective as
mupirocin ointment in the treatment of impetigo.
Based on the given data on that the most commonly isolated
microorganism is Streptococcus pyogenes and that the zone of
inhibition is seen in 5mg/mL, we used 5mg/mL extract of Curcumin
longa in the treatment of impetigo and folliculitis [24-26].
In this study, it was assessed that there is no significant difference
in the resolution of lesions after 2 weeks of application of Curcumin
longa 5% rhizome ethyl acetate extract in ointment as compated to
mupirocin 2% ointment. Twenty eight out of the thirty eight subjects
noted complete resolution of the lesions in the Curcumin longa
group. There was one hundred percent resolution of the lesions in the
mupirocin group. While the two-week recovery proportions between
mupirocin and Curcumin longa are statistically indistinguishable
from each other, we do see that mupirocin-treated lesions recover
significantly quicker than Curcumin longa-treated ones [27-30]. After
the first week, 72% of the mupirocin-treated lesions had recovered as
compared to only 31% for Curcumin longa-treated ones, with these
proportions being significantly different.
Conclusion
Our results showed that topical application of Curcumin longa
5% rhizome ethyl acetate extract in ointment is of comparable efficacy
and safety profile to mupirocin 2% in ointment in the treatment of
localized impetigo and folliculitis. However, mupirocin-treated
lesions recover significantly quicker than Curcumin longa-treated
ones. Generally, the results implied that at the end of 2 weeks, both
treatments had produced similar improvements on lesion counts.
The researcher recommends that on future studies on Curcumin
longa by increasing the sample size population, specifying the body
parts included, determining its efficacy against other microorganisms
(gram negative) and its use in other preparation (such as in cream)
and concentration.The author also recommends that there should
be a bacteriological examination of each patient diagnosed with
impetigo prior to application of the treatment to know specifically if
the Curcumin longa is more effective or equally effective for specific
species (Staphylococcus aureus or Streptococcus).