Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Two different Bioclinical profiles of Chronic Urticaria suggested by basophil number and Reactivity
Dzvig AC2, Lefevre MA1, Durieux C1, Manuel N3, Biron CA 3, Chazelle M3, Garcin A3, Perrot JL3 and Lambert C1,2*
1Immunology laboratory, University Hospital, France
2Allergology unit, University Hospital, France
3Department of Dermatology, University Hospital, France
4Department of Clinical research innovation and pharmacology (URCIP),University Hospital, France
*Address for Correspondence: Lambert C, Laboratoire d’Immunologie, pole de Biologie, Hop Nord CHU SaintEtienne,42055 Saint-Etienne Cedex 2, France; Tel: 33 477120513; Fax: 33
477120552; Email: claude.lambert@chu-st-etienne.fr
Submission: 22 May, 2021
Accepted: 24 June, 2021
Published: 30 June, 2021
Copyright: ©2021 Dzvig AC, et al. This is an open access article distributed
under the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original work
is properly cited.
Abstract
Chronic Urticaria (CU): is an heterogeneous disease supposed to be
due to spontaneous release of histamine from unclarified activation of mast
cell or basophil through IgE receptor pathway but treatment targeting either
histamine or IgE are not always successful. The aim of this study was to
explore Basophil phenotyping and functionality to better classify CU.
Methods: the prospective, clinical study enrolled 31 CU and 29 age and
sex matched controls. Basophils were analyzed by flow cytometry.
Results and Discussion: Our CU population demography was very
similar to cohorts previously reported. CU were active for 5.58+5.4 years and
severe (UAS7 = 25.8+10). Serum IgE were higher than 114kU/L in 36.8%
CU vs 12.0% HC. Serum tryptase was higher than 7µg/L in 20%. Basophil
represented less than 0.1% of leukocytes in 32.3% CU and even more in
case of recent flares. Ex vivo basophil activation was defective in 75.9% of
CU vs 31% of HC. However despite an active disease, 41.9% of patients kept
a high basophil count and 7 (24.1%) a high reactivity with low serum tryptase
and low activation profile suggesting their basophils are not the main effector
in the diseases.
Conclusions: Monitoring Basophil count and ex vivo reactivity together
with the level of IgE should help in suspecting the basophil and IgE involvement
or alternative mechanisms of CU. This could lead to a classification of CU in
its heterogeneity and help predicting its response to anti-histaminic or antiIgE
therapies.
Abbreviations
ASST: Autologous Serum Skin Test; FCM: Flow Cytometry;
MdFI: Median Fluorescence Intensity; Basophils Basophils; CU:
Chronic Urticaria; HC: Healthy Controls; BMI: Body Mass index;
tIgE: Total serum IgE; BAT: Basophil Activation Test; EDTA:
Ethylene Diamine Tetra Acetic Acid; PBS: Phosphate-Buffered
Saline; FITC: Fluorescein isothiocyanate; PE: Phycoerythrin; APC:
Allophycocyanin; NS: non-significant; Ig Immunoglobulin; NR ex
vivo Non-reactive (Basophils); FcεRI high affinity receptor for IgE;
IL3 Interleukin 3; UAS7: Urticaria Activity Score other 7 days; UCT:
Urticaria Control Test
Introduction
Chronic Urticaria (CU) is characterized by frequent, sudden
occurrences of transient itchy wheals and/or angioedema, over a
period of at least 6 weeks. Crises occur without any identified allergen
or exogenous triggers (spontaneous CU; CSU) unless it is triggered
by physical signals (inducible CU; CINDU) such as dermographism,
heat or cold contact, pressure or vibrations, water , sun or neuroendocrine
stress [1-3]. CU is frequently associated with atopy or
auto-immunity [4]. CU is more frequent in females for unclear reasons. CU can appear at any age and persist for 3-5 years before
disappearing. Approximately 1/5 cases persists for more than 5 years
[1,2]. More severe the disease is, longer it persists [5].
The diagnosis of CU is essentially clinical. The daily Urticaria
Activity Score over 7 days (UAS7) measures the disease activity/
severity, while the Urticaria Control Test (UCT) measures the
treatment efficacy [3,6]. CU is considered highly active when UAS7 is
higher than 27 and is poorly controlled by the treatment when UCT is
below 12. CU is frequently associated with obesity (Body Mass Index
-BMI- higher than 27) [7] and raise of D-dimer [8,5]. Obesity is
related to the patient age [9-11].
It is generally admitted that CU symptoms are due to inappropriate
mast cells and basophil degranulation. Accordingly, it was shown that
these cells infiltrate the wheal lesions [9,12] and that basophil count
is reduced during flares, as demonstrated long before Flow cytometry
(FCM) was used [13-15]. Basopenia is believed to be due to local
recruitment [16]. Histamine and tryptase raise may be detected in
serum during flares [17-19] and non-sedative anti-Histamine (H1)
drugs may relieve symptoms, but frequently require high doses. Total
serum IgE (tIgE) are usually in the normal range (<114kU/L) unless
the patient is atopic. IgE play an important role in mast-cell/basophil
homeostasis [20] and basophil degranulation is easily induced ex vivo
with anti-IgE or anti-IgE receptor type I (Fcε-RI) antibodies. AntiIgE
biotherapy (namely Omalizumab) has remarkable efficiency in
preventing CU’s flares in a great majority of the patients [21,22].
Anti-IgE biotherapy is known to prevent the IgE binding to mast
cells and basophil membrane Fcε-RI. So, IgE, Fcε-RI, degranulation
and release of histamine are considered as critical in inducing CU
symptoms but still, CU’s physiopathology remains unknown and not
all patients are sensitive to anti-HI or Omalizumab treatments.
CU may be an auto-immune disorder. Indeed, CU is frequently
associated with autoimmune diseases like thyroiditis. Furthermore, Skin Test with Autologous Serum (ASST) may induce wheals possibly
due to the presence of auto-antibodies ASST is not used in France for
ethical reasons. difficult toe for inter laboratory comparison [23,24].
Several arguments suggest that major targets of autoimmunity in CU
are either FcεRI or IgE most of which being bound to their membrane
receptors (named Type II Autoimmune CU). Unfortunately there is
no reliable method available yet to detect these auto-antibody, except
the ASST. Alternatively IgE auto-immunity to organs or tissue have
been suspected (named type I auto-immune CU or Auto-Allergy)
and IgE anti-thyroperoxidase, thyroglobulin and interleukin-24 have
been reported in CU [6,25-27].
The Basophil Activation Test (BAT) measures basophil
degranulation by FCM despite the low concentration of basophils
in peripheral blood. Basophils are identified by immunodetection
of membrane proteins such as CCR3, CD123 (together with
plasmacytoid dendritic cells) or CD203c. CD203c that is basically
expressed on basophils and is upregulated during stimulation.
Basophil granules express protein p53 (CD63) in their inner side
of the membrane, not accessible to immunolabelling of fresh
cells. Basophil degranulation induces a fusion of granules with the
cytoplasmic membrane and a strong and rapid expression of CD63
that become detectable on the surface of the cells [28]. Usually, less
than 5% of basophils spontaneously express CD63. Degranulation
is rapidly induced by allergenes in allergic patients. BAT sensitivity
is generally improved by IL-3 for diagnosis. using Furthermore,
a default in ex vivo basophils reactivity (PR) has been reported,
during sample preparation [15,29,30]. Basophil general reactivity is measured by challenging them with Fcsymbol
RI or IgE monoclonal antibody. Note that BAT is performed in
whole blood and elevated plasma IgE can compete with in anti-IgE
stimulation. Basophil reactivity can also be tested with N-formylmethionyl-leucyl-phenylalanine
(fMLP) a strong stimulant of a G
protein-coupled receptor (FPR1), independently of Fcε-RI. Generally,
anti FcεRI, anti IgE or fMLP induce degranulation of up to 90% of
basophils in a dose dependent manner. For unclarified reasons, few
patient’s basophils have a poor ex vivo reactivity (PR), even when IL3,
as opposed to Good reactive (GR). The “non-responder” term is
some time used but is a source of confusion because it is also used for
patients who are not sensitive to the treatment. In CU, PR frequency is
increased and is related to the disease severity [31]decreasing during
remissions [14,32]suggesting some causality link. During flares, some level of basophils activation has been reported on expression of CD69
[33], CD203c [34] or even some spontaneous expression of CD63 [23,33]. An indirect BAT assay has been proposed to reproduce ASST using patient serum and a donor basophils or a mast cell line but this
test has poor performances and strongly need for standardization
[23,24].
So, IgE, mast cells/basophils and histamine release are considered
to be critical in the physiopathology of CU. An inappropriate mast
cell degranulation can be intrinsic or extrinsic mechanisms that are
not all identified. Several auto-immune mechanisms targeting mastcell/basophils
are
suspected
but
difficult
to
evidence
and
only
explain
part
of
the
cases.
As
a
matter
of
fact,
treatments
targeting
histamine
[35-37]
or IgE [38-40] are frequently inefficient. As we regularly
explore basophil as part of allergy diagnosis, we thought measuring basophil reactivity could help in a better understanding of the role of
basophil /IgE regarding the heterogeneity of CU.
The aim of this study was to find Basophil phenotype and
functional parameters that could help in better classifying CU.
To address that question, we performed large biological study of
basophils in a prospective, monocentric, case control study of CU
out-patients.
Materials and Methods
Between March 2016 and September 2017 we performed prospective observational study on patients with CU and compared
them to age and sex matched healthy controls (HC) from the
melanoma preventive outdoor clinic in the dermatology department.
HC with previous history of inflammatory or allergic skin diseases
or urticaria were not included. The diagnosis of urticaria was based
on clinical history and physical examination according to European
Guidelines guidelines [3]. Patients were invited to participate to
the study the first day they were addressed to the clinic. Data were
collected on age, gender, IgE levels, and allergy (asthma, atopic
eczema, food allergy, and allergic rhinitis). The disease activity
was evaluated using UAS7 and UCT scores according to European
guidelines [3]. During the same time, age and sex matched healthy
controls (HC) who attended the clinic for detection of melanoma,
without any history of urticaria were informed of the study. Patients
and volunteers who have accepted to sign the informed consent were
enrolled and tested the same day.
Serum total IgE (reference value<114kU/L) and serum Tryptase
(reference value<11.4kµg/L) were measured using ImmunoCAP
(UNICAP 250, Thermo Fisher Scientific, Uppsala, Sweden). Serum
total IgE were calibrated between 2 and 5000 kUI/L as referred to the
international 75/502 WHO standard) and Tryptase was calibrated
between 1 and 200 µg/L. D Dimer values were measured using Vidas
D-dimer exclusion II DEX2 (Biomerieux Lyon France). D Dimers
reference values were below <500u.
Basophil phenotyping was performed on EDTA anti-coagulated
blood the day of enrolment. Basophils were identified using antiCD123 antibody conjugated with Allophycocyanin (APC)- Alexa 700 (clone SSDCLY107D2, Beckman-Coulter; Fullerton, CA), antiHLA
DR
Horizon-V450
(clone
L243,
BD
Biosciences®
San
Jose,
CA).
Basophil
activation
was
measured
using
BasoflowEx
kit (Exbio, Praha,
Czech Republic) adapted as described recently [28]. Briefly, EDTA
samples were diluted 1/1 with Hanks saline Buffer complemented
with calcium chloride 20µmol/ml (Renaudin laboratory, France) and
sodium heparinate 500 UI/ml (Choay; Sanofi-Aventis®, France). No
IL-3 was added in the test. Basophils were firstly either unstimulated
or stimulated with anti-IgE antibody (clone BE5, Exbio, Praha)
at10µg/ml or part of the cohort with anti-Fcε-RI 5 µL (provided in the
Bühlmann Laboratories AG kit, Schönenbuch, Switzerland) and with
anti-IgE +formyl-methionyl-leucyl-phenylalanin (provided in the
Exbio BasoflowEx
®
®
kit). The samples were stained with anti-CD203cPE
monoclonal antibody (clone NP4D6) and anti-CD63-FITC
(clone MEM-259, Exbio, Praha) during the activation time, for 20
minutes in the dark in a 37°C water bath. Erythrolysis was performed
using Immunoprep on TQ-PREP Workstation (Beckman-Coulter;
Fullerton, CA). At the end, the sample was washed in Phosphate buffer (PBS, Eurobio, France) and re-suspended in PBS-1% Bovine
serum Albumin fraction V (BSA, Eurobio Courtaboeuf France) and
analyzed within 4 hours on Navios
™
cytometre using Navios
software
(Beckman-Coulter).
Data were analyzed on Kaluza
software (Beckman-Coulter): cell
shape was checked on Forward/side scatter dot plots. Bubbles were
excluded on basophil label versus time scatter. Cell doublets were
excluded on Forward area under curve/ height scatter. Basophils
were then selected on their specific expression of CD203c versus
Side Scatter (SSC) and the fractions of activated basophils (CD63
™
)
as well as the CD203c/CD63 labelling (MdFI) were measured among
this population. Cytometer settings were checked daily using quality
controls procedure according to the manufacturer instructions. Compensations of spectral overlaps were set up using single labeling
on anti-mouse IgG beads (Versacomp
beads, Beckman-Coulter) and
calculated using the software application on Navios software.
Statistical Analysis
Correlations were analyzed using linear regressions, Student T
and chi² tests from Excel (Microsoft Corporation, Redmond, WA).
Comparison between groups was tested with the non-parametric Mann-Whitney or Kruskal-Wallis tests, while categorical variables
were assessed using the Chi-Square or Fisher’s exact tests using
GraphPad
software.
Ethical issue
All subjects submitted a written informed consent form at the
time of their enrollment into the present study. This biological study
was performed blindly on anonymous blood samples collected for
diagnosis purpose in accordance to 2011-814 bioethical French law.
Results
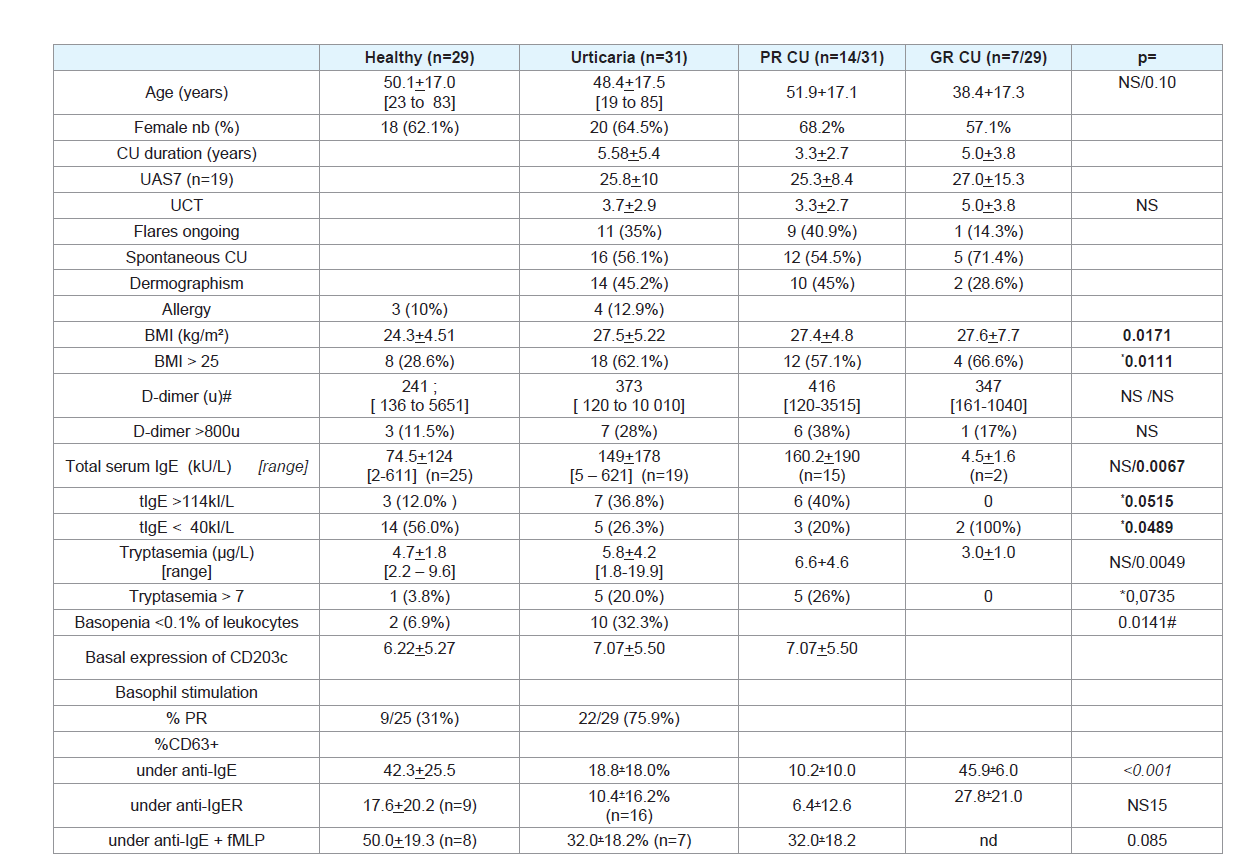
Our CU population demography was very similar to previously reported cohorts (Table 1):
Thirty one patients with CU were enrolled in the study, 20 were
females (65%) and 11 males, with a mean age of 48.4+17.5 years (from
19 to 85). CU patients were compared to 29 HC of which 18 (62%)
were females and 11 males with a mean age of 50.1+17.0 (from 23
to 83) years. Demographic and clinical features are summarized in
(Table 1). The patients claimed to have the disease active for 5.58+5.4
years; 21 patients (77.4%) had CU for more than 1 year, up to 21. The
UAS7 obtained from 19 patients only, reflected that CU was severe.The UCT score was reported by all but one patients and was lower
than 11 in 29 out of 30 patients, and below 5 in 63.3% of the patients.
Among 19 patients who provided both UCT and UAS7 scores, 84.2%
had a high UAS7 >27 and a low UCT<5.
Urticaria flares were induced (CINDU) in 15 patients, all
presenting dermographism and 4 of them having crisis triggered:
one by pressure, one by cold air, one by cold water and one by
hot air or stress. CINDU and spontaneous CU (CSU) had similar
distributions of age or sex, CU duration and BMI, UAS7 or UCT
scores. One CSU patient had a history of allergy to food while among
CINDU patients, one was allergic to pollens only, one to bee venom
and one, whom flares were triggered by cold water, was allergic to
animal fur and pollens. Comparatively, 3 HC had an allergy. Allergic
CU patients had similar ages, BMI, IgE, basal serum tryptase and
basophil status as patients without allergy. None of allergic patients
bothered reporting their UAS7 questionnaire. Because of their low
number, the whole CU (CSU, CINDU, with or without allergy) group
was analyzed altogether unless it is mentioned. Ten patients were not
treated before they enrolled in the study. Seven patients were taking 1
or 2 antihistamine tablets per day; 4 more were taking 3 tablets and 5
more were taking 4 tablets per day. Furthermore, 5 patients had taken
steroids at some time of the disease (all CSU), 1 had been treated with
methotrexate and 2 with montelukast.
CU patients had BMI (27.5+5.22 kg/m²) significantly higher than
HC (24.3+4.51, p=0.0171, (Table 1). Among CU patients, 62.1% had a
BMI >25 compared to 28.6% of HC (chi² p=0.0111) and 34.5% had a
BMI >30 compared to 14.3% of HC (p=0.077). D-dimer values were
widely distributed in the two groups and 28% of CU had D-dimer
higher than 800u as compared to 11.5% of HC (NS). D-dimer values
were not correlated to the UAS7 or UCT scores or to the BMI (Table 2). Patients with a BMI >30 tended to have higher D-dimer (median:
680±739) compared to patients with lower BMI (326±857, NS);
tended to be older (52.4±19.5 compared to 45.8±17.4 years, NS) and
having a longer disease duration (7.5±6.57 compared to 2.81±3.56
years, p=0.059), but a lower disease activity (UAS7 score: 23.5±10.7
compared to 26.2±10.1; NS). The UCT score, tIgE level, serum tryptase, basophil count and basophil reactivity were not different
between patients with moderate of very high BMI. Serum tIgE levels
were elevated (>114 kU/L) in 7 out of 19 CU patients tested (36.8%)
but only in 3 of 25 HC (12.0%; chi² p=0.0515).
A great part of patients shows biological signature compatible with basophil – IgE involvement:
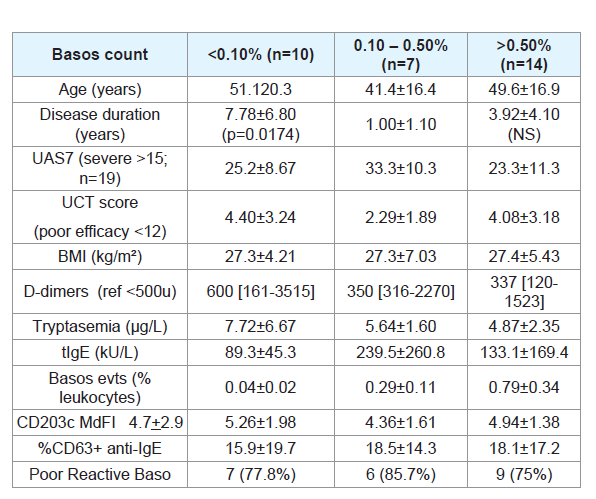
In 10/31 (32.3%) CU patients, the basophil count was low.
Because basophils are rare events among peripheral leukocytes
(<1%), we could not get highly reliable values for technically reasons.
Among cells expressing CD123, the mean number of basophils, that
expressed CD123 but not HLA DR-, was significantly lower in CU
(969+711 events) compared to HCs (1458+754 events, p=0.0192)
while plasmacytoid Dendritic Cells (pDC), that express CD123 and
HLA DR, were in similar numbers (438+478 in CU vs 404+290 in
HC, NS). Consistently, the mean ratio of basophil over lymphocyte
counts was lower (1.37+0.98%) in CU compared to HC (2.25+1.87,
p=0.0421). More simply, basophils represented less than 0.1% of
leukocytes named Basopenia (Bp), in 10/31 (32.6%) CU compared to
2/29 (6.9%) HC (ch² p= 0.0141). The difference was particularly high
in females CU patients, (40%) compared males (18%; chi² = 0.0004).In accordance with the hypothesis of local recruitment, basopenia
was more frequent in patients who reported recent flares. Patients
with Bp reported a longer history of CU (7.8±6.8 years) compared
to patients with a normal basophil count (1.0±1.1 years; p=0.0174)
and they tended to be older (51.1±20.3 vs 41.4+16.4 years old). Bp
was observed in 30% of patients with D-dimer higher than 800u but
in 10% of patients with D-dimer lower than 800U (ch²: p=0.0493).
Bp was not related to the BMI or UAS7 and UCT scores. However,
Bp patients tended to have lower IgE (89±45.3 vs 239.6±200.8) and
higher CD203c expression (5.97+1.95 vs 4.77+1.09) suggesting some
degree of basophils activation. Furthermore, in two cases, Bp was
associated with a raised serum tryptase (18.3+2.2 µg/L), a very long
story of the disease (>10 years) and a higher expression of CD203c
(MdFI: 7.23+3.42) on basophils suggesting a very active disease.
Basophil capacity for degranulation was frequently deficient
in CU (Table 1). Under stimulation with anti-IgE antibody, a poor
response of basophils (PR) was observed in 22/29 (75.9%) CU but
in 6/25 (31%) HC (chi² p=0.0031). Because anti-IgE stimulation
in whole blood competes with serum IgE that was elevated in a
few patients, we confirmed this defect in response by stimulating
basophils with an anti-Fcε-RI antibody in a part of patients. Anti-
Fcε-RI induced degranulation of 6.4+12.6% of basophils in 13 PR as
compared to 17.6+12.1% in 9 HC. Some kits provided and anti-IgE
completed with fMLP as positive control. Stimulation of basophils
with an anti-IgE + fMLP, induced a degranulation of 32.0+18.2 %
of basophils in 7 UC compared to 50.0+19.2% in 8 HC (p= 0.085).
PR patients tended to be older and had a longer CU history, a higher
UAS7 and a lower UCT; their tIgE levels were lower and their serum
tryptase higher (p= 0.0030).
On the other hand, some patients lacked evidence of peripheral basophil involvement:
Indeed, 14/31 (45.1%) CU patients kept a high number of basophils
(>0.50% of leukocytes) despite they had a very active disease. They
had low serum tIgE levels and low serum tryptase (4.71+2.33µg/L),
low expression of CD203c on resting basophils (MdFI: 5.64+3.97).These patients tended to be older (49.9+16.3 years) with a long disease
history (4.5+4.4 years) compared to patients who had a normal or
low basophil count. Their CU was more frequently associated with
dermographism (57%) compared to patients with normal (40%) or
low (43%) basophil counts. They had similar levels of BMI, D-dimer,
UAS7, UCT scores although they tended to take more tablets of
anti-H1.
Also, part of CU patients kept GR and low serum tryptase
(2.95+1.00µg/L; compared to PR; p=0.0049), even lower than HC
(6.05+5.86; p=0.028). GR patients generally had high basophil counts
with low expression of CD203c (4.94+1.38) but this group did not
completely overlap with the group of patients who had high basophil
count. These GR patients tended to be younger (38.4+17.3) than PR
patients (51.9+17.1, NS) despite they had a longer disease history
(5.0+3.8 years) and a high UAS7 score (27.0+15.3). CU was inducible
in 2/7 (29%) of GR as compared to 11/22 (50%) PR (NS). IgE dosage,
available in only 2 GR patients, was remarkably low (<10kU/L).
Discussion
This is the first description of French patient-control group
of patients with CU, in the real life. The population we observed
appeared to be very similar in age, higher frequency of females,
frequent dermographism and overweight distribution as other
populations described in other international [36,37,41-45] or French
[46,47] studies. The mean duration of the disease was quite long
but this does not mean it was due to a delayed diagnosis as most of
patients already have had 2 or 3 lines of treatment and were addressed
to the local reference center because of difficulties in controlling the
disease. This probably also explains why most cases were severe (high
UAS7, low UCT scores). As usually reported, CU was more frequent
in females for unclear reasons and we did not find differences between
females or males in terms of age, overweight, disease severity, IgE
levels, serum tryptase or basophil reactivity except that basopenia was
more frequent in females. It is a pity we miss information on possible
auto immunity because ASST is not used in France and there are no
standardized ex vivo alternative test that could replace it yet [48].
Like other studies, we also found a frequent association of CU
with overweight [10,36,37,41-47]. Not surprisingly, the overweight
was associated with age and disease duration as previously reported
[49]. Our data cannot tell if obesity was anterior and eventually
favoring CU or if it could have been a consequence of the disease
chronic inflammation. Hormonal disorders have been suggested
in epidemiological studies of CU [50] but gain of weight could also
be related to the chronic inflammation. Indeed, the production of
inflammatory cytokines has been associated with dysregulation of
appetite regulators like lipocalin-2 or adiponectin in CU [51,52]. In
any case, we did not evidence association between obesity and CU
severity in accordance to the previous report [11]. In our study, we
observed a very large variability of D-dimer levels in CU as well as
in HC. This is why we have reported results in median and range
values instead of mean and standard deviation. D-dimer is a marker
of coagulation disorder linked to the metabolic syndrome and
chronic inflammation [5,8,49,53,54]. In our experience, high levels
of D-dimer was not related to overweight but tended to be higher in
PR patients suggesting an effect of the disease activity and probably
the inflammatory status although anteriority in steroid treatment can
also play a role in it.
We analyzed serum tryptase as a marker of mast cell activation.
Raise of serum tryptase is very helpful in diagnosis of acute urticaria
and mast cell disorders. In our study, serum tryptase was rarely
elevated above the reference range in CU and was not correlated
to the disease severity. However, we could see that basal serum
tryptase, was still a bit higher in CU as compared to age, sex matched
HC in accordance with previous M Ferrer’s report [17]. In fact, the
reference ranges that are usually admitted, have been proposed by
the manufacturer of the dosage system but are certainly too broad.
Indeed, usual basal serum tryptase we routinely detect are closer to
the level we observed in our HC group. Considering the threshold
of 7 as a reference range, we observed that serum tryptase was more
frequently high in CU, and was more frequently high in patients who
reported recent flares just before they came to the clinics suggesting it
reflects some residual mast cell activation.
Beside the clinical data, we looked for biological parameters
theoretically related to the physiopathology that could help in
characterization of CU and although no basophil biological monitoring
is actually recommended in the international guidelines for the
diagnosis of CU. And indeed, we found that signs of involvement of
basophils and IgE were altered at least in part of patients. Basopenia is
compatible with basophil recruitment and consumption in the tissue
and basophil renewal can decline with the duration of the disease
[14,15,55,56]. Interestingly, Eosinopenia has also been reported in
CSU and has been associated with autoimmunity (ASST), low IgE,
basopenia and poor response to Omalizumab [57]. So basopenia is
an informative parameter to consider in monitoring CU activity (if
not severity) and is easy to get in routine differential leukocyte count
in accordance with Borzova [58]. Peripheral basophils show some
level of activation evidenced by a raise of CD203c expression but this
was limited in blood, probably because the cell activation is mostly
restricted to the tissue. Frequent challenges of basophils can explain
some level of exhaustion and lack of ex vivo reactivity that Sabroe
called “desensitization” [25,49]. Alternatively, PR can be explained in part by a lack of peripheral basophils maturity due to an increased
turn over.
Mast cell activation can explain the occasional raise of serum
tryptase. This was rarely observed, probably because the disease
history is so chaotic that it is difficult to get testing at the peak of
production in outpatients.
CU has been associated with a background of atopy that can
explain the high level of serum tIgE. However, if the eventual Anti-IgE
auto-immunity that eventually induces spontaneous degranulation
without requiring allergen [60], most probably also binds to soluble
IgE, making immune complexes rapidly removed from serum. This
can explain IgE depletion [61] while anti-FcRI auto-immunity
induces degranulation [61,62], but without interfering with soluble
IgE [63-66]. It is then not surprising to observe low IgE levels in some
patients but this can also depend on the chaotic appearance of flares
raising once more the high heterogeneity of the biological status
collected at some limited time points.
Daily dosages of tIgE in healthy population show a large variability
and are mainly considered as pathological when tIgE are above a
threshold (>114kU/L) that was defined by the major manufacturer of
the dosage system. Thus, dosage of IgE is rarely explored in its lower
range where its clinical significance is generally neglected. Our results suggest that considering lowering level of IgE could be of interest in
monitoring CU. Furthermore, one can question the potential benefit
of treatment with anti-IgE biotherapy in case serum tIgE are very low.
On the other hand, CU with high levels of IgE may be compatible
with a possible type I auto-immunity in which IgE may play a role
and high IgE has been shown to be associated with a good efficacy of
Omalizumab treatment of CU [39,67].
But our results also show the lack of evidence for basophile
involvement in some cases of CU. This suggests that CU could be
related to alternative release of vasoactive mediators, independent of
the IgE and Fcε-RI activation pathway as previously shown [66,68].
It is of interest to mention that CU has been observed in patients
with very rare primary deficiency in IgE [69].This would explain
some failure of anti-histaminic treatment. We cannot say if this is
compatible with the type I auto immune mechanism of CU as we
unfortunately lack of diagnostic tools for mechanism.
Conclusion
In conclusion, we report basophil monitoring in a prospective
case-control study of Chronic Urticaria diagnosed in a middle size
teaching hospital and our results show that our epidemiological data
are very similar to other studies reported. Our biological data show
that total serum IgE and serum tryptase dosages, basophil count
and basophil ex-vivo reactivity could bring precious information in
classifying CU according to different possible physio-pathological
mechanisms and could eventually anticipate the potential response
to biotherapy.
Acknowledgement
The authors gratefully acknowledge all participants who
volunteered for the study.