Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Evaluation of A Natural Anti- Dandruff Technology in a Shampoo Formulation via In-vivo and In-vitro Methods
Ahmed H1*, Diaz I1, Cai C2, Yin H2, Zuniga A3 and Sandoval F3
1Colgate Palmolive Company, Piscataway, United States
2Colgate Palmolive Company, Guangzhou, China
3Colgate Palmolive Company, Mexico City, Mexico
*Address for Correspondence:
Ahmed H, Colgate Palmolive Company, Piscataway, United States;
E-mail: hameda_ahmed@colpal.com
Submission: 24 January, 2023
Accepted: 27 February, 2023
Published: 06 March, 2023
Copyright: © 2023 Ahmed H, et al. This is an open access article
distributed under the Creative Commons Attri-bution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Abstract
Background: Natural anti dandruff efficacy is an area of interest as
there is a consumer need for natural technologies in over-the-counter
shampoo formulations.
Aim: This research explored dipotassium glycyrrhizate (DPG)
for anti dandruff benefits utilizing in vitro and in vivo methodologies,
examining a shampoo formulation with 0.15% DPG.
Patients/Method: The in vitro investigation focused on the
evaluation of antimicrobial efficacy of DPG in a shampoo formulation
by use of bacteriostatic efficacy testing as well as a short interval
kill test against well known dandruff causing microorganism i.e.
malassezia. Clinically, 28 female and male subjects possessing visible
dandruff were enrolled in a non-comparative study with 1 shampoo
formulation containing 0.15% DPG. A trained investigator conducted
visual technical assessments evaluating dandruff intensity at baseline
and after product use over 2 weeks. A product performance self-assessment
questionnaire was also completed for all subjects. The
clinical was conducted under the supervision of a dermatologist.
Results: Shampoo formulation with 0.15% DPG showed
bacteriostatic efficacy against well known dandruff causing
microorganisms i.e. malassezia. The short interval kill test shows that
a shampoo formulation with 0.15% DPG is more effective against
malassezia furfur (ATCC 14521) compared to the placebo, a non-anti
dandruff shampoo. Clinically, a shampoo formulation with 0.15% DPG
significantly reduced visible flakes/dandruff after 1, 7 and 14 days of
use from baseline and the greatest improvement as compared to
baseline was seen at week 2 (P < 0.001) with 90% improvement in
reduction of visible flakes/dandruff. The self-assessment questionnaire
results correlated to the visual technical assessment results.
Keywords
anti dandruff; malassezia; dpg; dipotassium glycyrrhizate;
natural active; shampoo; healthy scalp; scalp care; scalp microbiome
Introduction
The scalp consists of a diverse microbiome which contributes to
either a healthy or diseased scalp. For instance, the proliferation of
certain fungi in the scalp has been linked to seborrheic dermatitis
and dandruff. Seborrheic dermatitis generally affects the scalp, face
and other areas of the body where high concentrations of sebum are
found. In regards to dandruff, this is mainly found in the scalp where
it may cause scaling and itchiness with or without inflammation.
malassezia spp. is microorganisms well known for being the main
drivers for dandruff or seborrheic dermatitis [1]. Most of the species
in the genus malassezia are present in human skin where the
environment is warm and moist. Based on the genomic sequence
analysis of malassezia, this genus is understood to not have the gene
necessary for fatty acid synthesis. It contains lipase phospholipase genes instead; this acts on sebum in human skin which in turn releases
diglycerides as well as unsaturated and saturated fatty acids on the
scalp. Most of these fatty acids are consumed by the fungus for its
own growth on the scalp. This becomes a vicious cycle of malassezia
growth, release of fatty acids, consumption of those acids, and further
growth. In those individuals with sensitive skin where there may be
a concern in the skin barrier integrity, the fatty acids can penetrate
the stratum corneum and cause further irritation and inflammation,
ultimately producing various levels of dandruff and leading to other
scalp conditions [11-13].
In today’s market, the traditional actives that are found in
anti dandruff shampoo products are zinc pyrithione (ZPT) [11-12], climbazole [13] and piroctone olamine. There is extensive
clinical research and proven efficacy backing these ingredients
[15]. While they are efficacious, all three of these active ingredients
in formulations have their own set of challenges in a world that is
gearing toward a natural solution. For example, these ingredients
can provide an initial relief to a dandruff sufferer by eliminating or
reducing Malasseiza from the scalp, but this can lead to a further
imbalance in the microbiome due to the strength and impact of these
active ingredients on the scalp. Beyond the efficacy, anti dandruff
formulations tend to not be well-liked by consumers because of a
less than ideal effect on hair condition [11-13]. This can impact the
continued use of the product by the consumer, which if stopped
or switched to non-anti dandruff shampoo, can lead to a relapse of
dandruff and inflammation.
With that in mind, the aim of this research was to evaluate an
alternative active ingredient that falls in the realm of natural - a gentle
ingredient that would not be harsh on the scalp stratum corneum
while also providing relief from dandruff. DPG comes from licorice, a
plant originating in Asia, North Africa and Europe, belonging to the
Glycyrrhiza genus that comprises multiple species. Constituents of
Glycyrrhiza glabra are well known ingredients in a variety of medicinal
preparations, either in the ayurvedic medicine or traditional remedies
where multiple benefits are associated with the full plant consumption,
its roots or extracts derived from a specific part of the plant. The
benefits reach as far as antioxidant, anti asthmatic, anti diabetic, and even skin whitening activity has been attributed. Among the multiple
phytochemicals present in Glycyrrhiza glabra root, the DPG is known
for its anti-inflammatory and antimicrobial properties [8-10]. The
active constituents of Glycyrrhiza glabra, found in DPG, have been
documented as effective against fungi and bacteria [14]. This leads
us to focus on DPG in a shampoo formulation. This research helps
to continue the conversation on the scalp and dandruff, including
exploration of the use of a shampoo formulation with DPG to reduce
dandruff.
Materials & Methods
In Vitro Methods:
Bacteriostatic Efficacy [3]: malassezia furfur ATCC 44344
(test microorganism) was prepared in suspension. A stock of
microorganism culture was taken from the freezer and a small
amount of the frozen material was scraped from the surface with an
inoculating loop and inoculated onto a Lecithin Tween-80 Nutrient
Agar (LNA) plate. The plates were incubated at 36°C for 5-7 days. A
single colony was transferred from the plate with an inoculating loop
to spread on a freshly prepared LNA plate. After incubation (36°C for
5-7 days), the microorganism was washed with 0.85% saline solution.
The level of initial microorganism suspension was about 1.0×105
CFU/ml. This was vigorously vortexes.5.0 ml diluted shampoo sample (1:10 dilution with standard
sterile hard water) was pipette into a tube, then kept at 20°C for 5
min. 0.1 ml of microorganism suspension was added to the tube
containing 5.0 ml of diluted shampoo. The shampoo was vigorously
mixed with the microorganism suspension thoroughly. At certain
time points (30 s, 1 min, 1.5 min, 2 min, 5 min, 10 min, 1 h, 2 h, 3 h,
4 h, 5 h, 6 h), 0.5 ml of the mixture of microorganism suspension and
shampoo was transferred to a tube containing 4.5 ml of Phosphate
Buffered Saline (PBS). This was vigorously vortexed. After 10 mins,
serial 10-fold dilutions with 0.85% saline were made and thoroughly
mixed by vortexing; an aliquot of 1 ml was pipette into a plate. 15-20
ml melted LNA agar (maintained at 44-48°C) was poured into the
plate. The plate was rotated several times to disperse the product
dilution. The plates were inverted and incubated at 36°C for 5-7 days
after the agar was solidified. The colonies were counted to calculate
the survival microorganism and bacteriostatic rate [3].
For the positive control, PBS was used instead of shampoo and
the above steps were repeated.
Acceptance criteria
Bacteriostatic rate (%) = (I-II)/I*100.
I-Control sample colonies
II-Tested sample colonies
Bacteriostatic rate ≥50%~90%, indicates test sample has
antibacterial effect
Bacteriostatic rate ≥90%, indicates test sample has strong
antibacterial effect
Short Interval Killing Test (SIKT) [4-7]: 4.5 g of each test
sample was aseptically weighed into sterile homogeneous bags. 4.5
mL of sterile DI water was aseptically pipette into a test tube to serve
as a test control.
Each sample was inoculated and controlled with 0.5 mL of
prepared malassezia furfur ATCC 14521 (approximately 106 CFU/L).
It was mixed properly with a wooden applicator. Timer started
immediately after inoculation. At the end of the selected time point (1
min, 2 min, 5 min), 0.5 ml of inoculated sample was added to 4.5ml
of Dey/Engley (D/E) broth and vortexes until properly homogenized.
This tube was denoted as 10-1 dilution and made dilutions up to 10-5
(performed dilution up till 10-7 for Negative control) in D/E broth
and plate 1ml of each dilution in petri plate in duplicates, add 15-20
mL of melted Dixon Agar.
Plates were incubated at 30°C for at least 2-5 days. After
incubation, plates were read and results recorded at 48hrs (2 days)
and then at day 5 [4-7].
The higher kill rate and larger Log reduction indicate the better
performance.
In vivo Method:
30 female and male subjects with visible dandruff 18-56 years
of age were enrolled in a clinical study. Following completion of an
Independent Ethics Committee (IEC) approved informed consent
(CONEP) and after meeting all inclusion criteria and none of the
exclusion criteria, subjects with a minimum sum score of 10 points
on the whole head through an dandruff intensity assessment done
by a trained technician were enrolled in the study. Subjects were not
allowed to use other anti dandruff products throughout the course
of the study aside from the study product. The study was conducted
under the supervision of a dermatologist.All subjects who participated in the clinical research signed an
Independent Ethics Committee (IEC) of Investiga - Instituto de
Pesquisa, registered by the National Research Ethics Commission
(CONEP) approved consent, and Good Clinical Practice guidelines
were followed. The study was conducted in compliance with the
Declaration of Helsinki principles.
A daily application of the test product (shampoo formulation
with 0.15% DPG) was completed by a trained technician five (5) times
a week on the weekdays for 2 consecutive weeks for each subject.
Subjects were provided with the test product (shampoo formulation
with 0.15% DPG) and instructed to use on the weekends at home
following use-instructions learned at the facility. Subjects were
allowed to continue the use of their own standard conditioner for the
duration of the study.
The design was a non-comparative study, where the test product
was tested on the subject’s scalp/hair. On the initial visit (D0), after
the visual technical assessment, an image was captured of the scalp
before product use. Then, a controlled application of the test product
was performed for each subject following a standardized method at
the facility by a trained technician. After 24 hours (Day 1), week 1
(Day 7) and week 2 (Day 14), with continued daily application of the
test product, new visual technical assessments and images of the scalp were captured via a Nikon camera with a Canfield Epiflash system
(Canfield Scientific, Parsippany, New Jersey, USA). The images were
captured under standard lighting from the top view of the scalp which
was divided into four (4) quadrants i.e. five images were captured for
each subject, one for each quadrant and one top view image during
each evaluation. At the completion of the study, a self-assessment via
questionnaire was completed by each subject.
Washings followed normal use instructions by applying 5-10 ml
of test product depending on hair length, washing/massaging the
hair with the test product for 60 seconds and rinsing for 60 seconds
followed by application of the subject’s standard conditioner.
Thirty (30) subjects were enrolled in the study and 28 twentyeight
subjects completed the study. One subject voluntarily withdrew
and was discontinued from the study. Separately, there was one
adverse event reported in which the subject experienced pruritus and
scaling due to an individual sensitivity; the subject was voluntarily
withdrawn from the study.
Statistical significance was defined as P-value less than or equal
to 0.05.
The results of the self-assessment questionnaire at the end of
the study were analyzed with a Binomial Test for within treatment:
comparisons between frequencies of top box evaluations (2, 1) and
bottom box (-1, -2) evaluations were performed. A significance level
of 0.05 (5%) was chosen for all statistical analysis. Statement 3 of selfassessment
correlates directly to the visual technical assessments i.e.
improvement in dandruff.
Results and Conclusion
In vitro results:
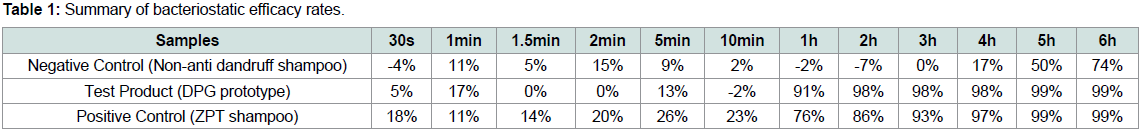
In Table 1, the bacteriostatic efficacy rates of the test product
and controls (negative control: non-anti dandruff shampoo, positive
control: anti dandruff shampoo with ZPT) prior to the 1 hr time point
were low (< 24%). After 1 hr, the bacteriostatic efficacy rates of the
test product and positive control showed high antibacterial efficacy,
>76%, as compared to the negative control, <1%. This indicates that
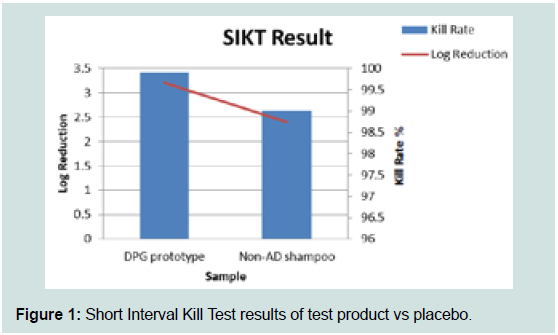
the test product is parity in efficacy to the positive control.In Figure 1, the SIKT results show that the log reduction of test
product versus water is 3.2, correlating to a 99.9% bacteria kill rate.
The log reduction of placebo versus water is 2.4, correlating to a 99% bacteria kill rate. The test product is significantly better (1 log
reduction) in achieving antibacterial efficacy when compared to the
placebo.
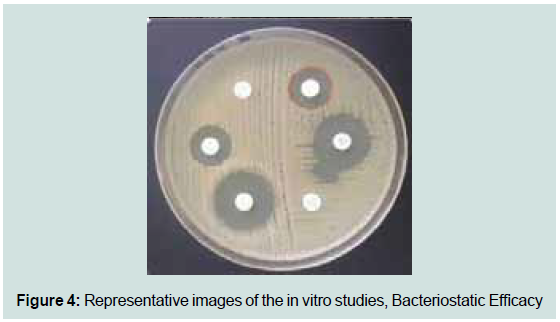
See Figure 4 for representative images of the culture plates for
these evaluations. The results show a reduction of malassezia after
exposure to the test product.
In vivo results:
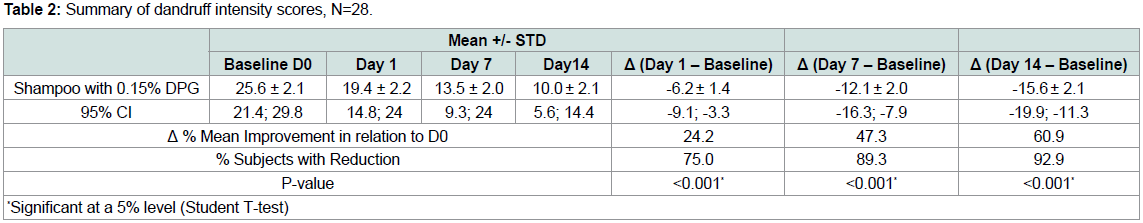
The results show (Table 1) that shampoo with DPG was
significantly effective in reducing dandruff/visible flakes at all timepoints
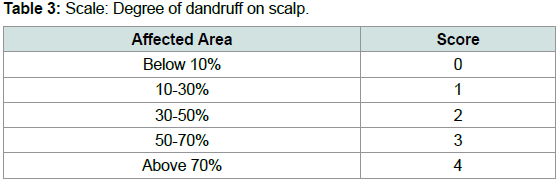
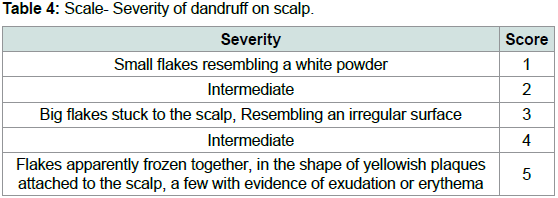
(p<0.05).Table 3 and Table 4 are scales used to score the degree of dandruff
of the scalp in each quadrant for each subject and the severity of
dandruff in each quadrant for each subject. Scores from both scales
were used to calculate the overall intensity of dandruff in the scalp for
each subject [2].
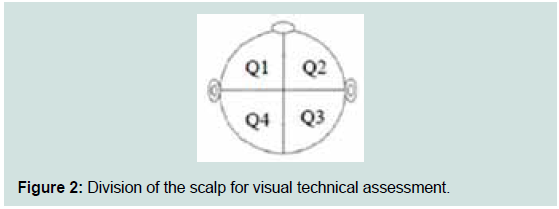
To calculate the intensity of dandruff for each subject, scales were
used for scoring and a calculation after dividing the scalp into four (4)
quadrants as seen in Figure 2.
Calculations:
Intensity of dandruff of the right side (Q2 and Q3) = (upper
right score of Degree of Dandruff X upper right score of
Severity of Dandruff) + (Lower right score of Degree of
Dandruff X lower right score of Severity of Dandruff)
Intensity of dandruff of the left side (Q1 and Q4) = (upper left
score of Degree of Dandruff X upper left score of Severity of
Dandruff) + (lower left score of Degree of Dandruff X lower
left score of Severity of Dandruff)
Total intensity of dandruff per subject = Intensity of dandruff
of the right side (Q2 and Q3) + Intensity of dandruff of the left
side (Q1 and Q4)The questionnaire evaluations were summarized by counts
and percentages of scores based on questions asked in Tables 5-7.
Further, the categories of disagree (-1, -2), agree (2, 1), and neutral
(0) questionnaire answers were given accordingly.
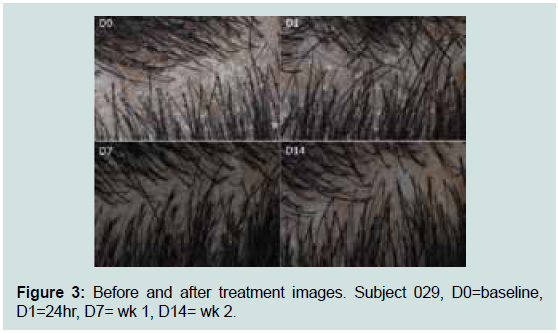
Visual images were captured by a Nikon camera with the Canfield
Epiflash system of subjects 029, 056, and 033 from the study (Figure 3 and 4). Results show a significant reduction in visible flakes.
Figure 3: Before and after treatment images. Subject 029, D0=baseline,
D1=24hr, D7= wk 1, D14= wk 2.
Conclusion
In vitro testing confirmed the antimicrobial efficacy of
dipotassium glycyrrhizate at 0.15% in a shampoo formulation
through bacteriostatic efficacy testing and short interval kill test. The
results for the short term interval kill test showed that the test product
performed better in eliminating malassezia when compared to the
negative control (placebo).
Clinically, the shampoo formulation with 0.15% DPG significantly
reduced visible flakes/dandruff after Day 1, 7 and 14 of use from baseline and the greatest improvement as compared to baseline
was seen at week 2 (P < .001) with 90% improvement in reduction
of visible flakes/dandruff. The self-assessment questionnaire results
correlated to the visual technical assessment results.
This study explored the use of DPG as a natural solution to
dandruff and over all, helped to gently prevent the scalp from
dandruff proliferation.
Based on preliminary research, literature and the series of tests
as captured in this paper, we hypothesize that there are four (4) key
factors that provide anti dandruff benefit from a DPG shampoo
formulation. First, it’s documented in various publications as
mentioned previously, that DPG provides anti inflammatory and
antimicrobial benefits [14]. Secondly, the complete formula is at
a pH of 4.0 which provides a mild acidic environment in order to
inhibit bacterial and fungal growth. In addition, the formula contains
a high level of surfactants which helps to enhance overall cleansing
as well as antibacterial and antifungal benefits. Lastly, the formula
contains menthol which provides a soothing feel as to prevent itching
of the scalp. The synergy of the above factors delivers significant anti
dandruff benefits.
A limitation in this research is that the clinical study did not
contain a comparative control to help distinguish any benefit over
traditional anti dandruff shampoos. In addition, the evaluations were
restricted to only dandruff and flaking. To continue understanding
the impact of natural ingredients such as DPG on the scalp in a
clinical setting, future research should include a control test product
as well as evaluations such as anti inflammatory biomarkers and
microbiome shifts from baseline to after product use. This research
can be used as the foundation work for future research in the area of
scalp health as related to the scalp’s microbiome via metagenomics
and sequencing [17].
Acknowledgements
The authors wish to thank Mariane Martins Mosca from Allergisa
Pesquisa Dermato-Cosmética LTDA, Brazil who was the Principal
Investigator in conducting the clinical study as well as Inolex, China
who supported the bacteriostatic efficacy testing. The entirety of this
research was funded by Colgate Palmolive Company, USA.
Conflict of Interest:
This research was funded by Colgate-Palmolive and all of the
authors are employed by Colgate-Palmolive.Author Contribution:
All authors participated in the conduct of the research and writing
of the manuscript.Ethical Approval:
All subjects who participated in the clinical research signed an
Independent Ethics Committee (IEC) of Investiga - Instituto de
Pesquisa, registered by the National Research Ethics Commission
(CONEP) approved consent, and Good Clinical Practice guidelines
were followed.