Journal of Clinical and Investigative Dermatology
Download PDF
Research Article
Clinical Outcome of the Topical Application of a Novel Hair Growth Ingredient, USPlus DERM, in Men and Women
Hill WS, Bousquet M, Carletto C and Dohnalek MH
1U.S. Nutraceuticals, Inc., dba Valensa International, 2751 Nutra Lane,
Eustis, FL 32726 USA.
2SYRES Sensory & Consumer Research,, 4 rue de Gally, 78450 Chavenay, France.
3Cosmeto Azur Consulting for VENDEIS sarl, 244 Ter Avenue Georges Pompidou, 06220 Vallauris, France.
2SYRES Sensory & Consumer Research,, 4 rue de Gally, 78450 Chavenay, France.
3Cosmeto Azur Consulting for VENDEIS sarl, 244 Ter Avenue Georges Pompidou, 06220 Vallauris, France.
*Address for Correspondence:Margaret H. Dohnalek, PhD, Chief Science Officer, U.S.
Nutraceuticals, Inc. dba Valensa International, 2751 Nutra Lane,
Eustis, FL 32726 USA. Email Id: m.dohnalek@valensa.com
Submission: 10 January, 2025
Accepted: 27 January, 2025
Published: 31 January, 2025
Copyright: © 2025 Hill WS, et al. This is an open access article
distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in
any medium, provided the original work is properly cited.
Keywords: Androgen-Mediated Hair Loss; Dermatology;
Dihydrotestosterone; 5α-Reductase; Free Fatty Acids; Hair Growth; Hair
Loss; Lipidosterolic Extract; Scalp
Abstract
Background:USPlus® DERM Topical is a proprietary lipidosterolic
extract of saw palmetto concentrated in key bioactive free fatty acids
important for hair structure and hair characteristics, and with potent
activity against 5α-reductase 1, to support hair follicle growth and hair
regrowth.
Methods:Adults aged 25-65 years experiencing unresolved hair loss for ≥6 months participated in a 12-week blinded clinical study evaluating the daily application of a 2% serum of USPlus DERM on the scalp. Dermatologic assessments were made at baseline and after 12 weeks of use, while participants reported their impressions at baseline and after 4, 8, and 12 weeks of use.
Results:Twenty-two participants (15 male, 7 female) completed the study. At 12 weeks, dermatologic assessment found significant improvements in hair loss for men (8.7%, P=0.009) and women (25.2%, P=0.004), with dandruff also less visible (31.7% decrease; P=0.047). Within the first 4 weeks of use, 59.1% of participants agreed that the product appeared to stimulate hair growth. After 8 weeks, participant assessment of general hair loss and density improved by 18.4% and 10.2% (P=0.129 and P=0.007, respectively), and hair loss when styling decreased by 28.9% (P=0.013).At study end (12 weeks),the majority of participants (90.9%; P=0.0001) noticed an improvement in hair loss/density. General hair loss and density improved by 22.4% and 19.5% (P=0.067 and P=0.001, respectively) and hair loss when styling decreased by 34.2% (P=0.005). Participant-rated product satisfaction was a mean 7.7/10, compliance with use was high, and the product was well tolerated.
Conclusions:Over 12 weeks, participants with a range of hair and skin types experienced a significant improvement in hair loss and hair density with the use of USPlus DERM Topical serum over 12 weeks. The proprietary USPlus DERM extract shows promise in supporting healthy hair growth and characteristics.
Methods:Adults aged 25-65 years experiencing unresolved hair loss for ≥6 months participated in a 12-week blinded clinical study evaluating the daily application of a 2% serum of USPlus DERM on the scalp. Dermatologic assessments were made at baseline and after 12 weeks of use, while participants reported their impressions at baseline and after 4, 8, and 12 weeks of use.
Results:Twenty-two participants (15 male, 7 female) completed the study. At 12 weeks, dermatologic assessment found significant improvements in hair loss for men (8.7%, P=0.009) and women (25.2%, P=0.004), with dandruff also less visible (31.7% decrease; P=0.047). Within the first 4 weeks of use, 59.1% of participants agreed that the product appeared to stimulate hair growth. After 8 weeks, participant assessment of general hair loss and density improved by 18.4% and 10.2% (P=0.129 and P=0.007, respectively), and hair loss when styling decreased by 28.9% (P=0.013).At study end (12 weeks),the majority of participants (90.9%; P=0.0001) noticed an improvement in hair loss/density. General hair loss and density improved by 22.4% and 19.5% (P=0.067 and P=0.001, respectively) and hair loss when styling decreased by 34.2% (P=0.005). Participant-rated product satisfaction was a mean 7.7/10, compliance with use was high, and the product was well tolerated.
Conclusions:Over 12 weeks, participants with a range of hair and skin types experienced a significant improvement in hair loss and hair density with the use of USPlus DERM Topical serum over 12 weeks. The proprietary USPlus DERM extract shows promise in supporting healthy hair growth and characteristics.
List of abbreviations
5αR = 5α-reductase; DHT = dihydrotestosterone; FFA = free fatty
acid; HRQoL = health-related quality of life; LSESr = lipidosterolic
extracts of Serenoa repens (saw palmetto); PHL = pattern hair loss;
SD = standard deviation.
Introduction
The hair loss treatment market was valued at USD $20.5 billion in
2023 and is projected to grow at a compound annual growth rate of
7% to $32.9 billion by the end of 2030 [1]. Hair loss is typically related
to one or more of the following factors: genetics, hormonal changes,
underlying health issues or medication use, stress, or due to hair
follicle damage, such as from chemical treatments [2]. Androgenetic
alopecia, or pattern hair loss (PHL), is the most common form of
hair loss [3].By age 50, PHL affects one-half of men and one in five
women [4,5]. Androgens are involved in normal hair growth, but
can lead to PHL when excess conversion of testosterone to the more
potent metabolite, dihydrotestosterone (DHT) occurs at androgen sensitive
areas of the scalp via the 5α-reductase (5αR) enzyme [5].
Increased DHT production and elevated levels of androgen receptor
sites on hair follicles affect the hair growth cycle and lead to follicular
miniaturization and thinning [6].
Hair loss can be addressed with short-term medical treatment or
the withdrawal of a causative medication, but PHL is a progressive
condition that overlaps both medical and aesthetic considerations [2,7].
A number of effective treatments are available for PHL, including
pharmacologic and natural therapeutics [2,4]. For example, the 5αR
inhibitor drugs have been shown to reverse hair loss; finasteride targets
the type 2 5αR isoenzyme, while dutasteride targets both type 1 and
type 2 5αR isoenzymes [2,8]. But some patients avoid or discontinue
pharmacologic agents due to concerns regarding long-term treatment
and/or side effects [6,9]. Drug costs can also be a barrier [6]. These
patients may prefer natural treatment options, including plant-based
topical oils (ie, pumpkin and tea tree); proprietary nutraceutical
mixtures and marine complexes; and lipidosterolic extracts of Serenoa
repens (LSESr), also known as saw palmetto [1,4,6].
Clinical studies have shown that saw palmetto extract, used
as a combination or single-ingredient product for oral or topical
application, can address hair loss [9-17].Additionally, this treatment
has been associated with only minor side effects, and patients have
reported high rates of satisfaction[9,12-17]. LSESr have been shown
to be potent inhibitors of 5αR 1 and 2 [18-20] and address PHL via
a mechanism that is similar to 5αR therapeutics [21]. LSESr extracts
have a unique fatty acid profile [22], and the quality, quantity, and
distribution of free fatty acids (FFA) in LSESr drives their ability to
inhibit 5αR enzymes [19,20]. As the most common lipids in hair [23,24], FFAs are highly bioactive and are taken up rapidly at androgen
receptor sites, including the skin and scalp [24,25]. FFAs coat and
protect the hair shaft [23], are essential to follicle structure [26], and
help regulate hair health and the hair cycle [24,27].Animal models
have shown that, after oral administration, available bioactive FFAs
such as those found in LSESr quickly localize to androgen receptor
sites, such as the skin and scalp [25]. where hair follicles are located.
The potency of LSESr products, however, varies substantially due to
differences in FFA profiles; specifically, the levels of bioactive FFAs
[18-20].
USPlus® DERM (Valensa International; Eustis, FL) is a novel,
proprietary LSESr extracted to naturally enrich the level of FFAs and
deliver a highly concentrated ratio of the four most bioactive FFAs
shown to help address hair loss and support hair regrowth [25,28-30]. Extracted using a proprietary ultra-high pressure carbon dioxide
process, this standardized quality ingredient meets the United States
Pharmacopeia Monograph for potency, purity and authenticity.
USPlus DERM has established biological activity to strongly inhibit
5αR enzymes 1 and 2 [18] and has been shown to promote hair
growth ex-vivo in healthy human hair follicles [31].
Study Objective:
This quantitative, blinded observational consumer-use pilot
study evaluated the efficacy (hair loss and regrowth), tolerability, and
acceptance of USPlus DERM Topical, applied as a 2% serum to the
scalp in adult men and women over three months of use (March 18-
June 9, 2024).Materials and Methods
Participants and Study Design:
Participants were from the Paris, France region, identified from
a database of panelists participating in consumer use tests (SYRES
Sensory & Consumer Research; Chavenay and Paris, France).
Participants were initially pre-selected based on being in the target
age group of 25 to 65 years. The 210 pre-selected participants were
screened by phone during February and early March 2024 with a 33-
item questionnaire to assess eligibility, hair care product use history,
hair characteristics, and hair and scalp condition. For this pilot study,
a minimum enrollment of 22 men and 10 women was needed to
achieve the study criteria for a minimum of 15 male and 5 female
completers, with balanced representation across the age ranges of
25-35, 36-45, 46-55, and 56-65 years. All eligible participants were
referred for a baseline dermatologist evaluation, conducted on 18
March 2024 at SYRES Sensory & Consumer Research in Paris. A
study timeline is shown in (Figure S1).The clinical study protocol allowed for the inclusion of participants
with all hair types and hair and scalp conditions, including dandruff.
To be included, participants were required to have experienced hair
loss for at least 6 months, ranging from slight to significant or chronic.
Participants agreed to use the hair growth serum at home for the next
12 weeks according to instructions, and to commit to being available
for the full study period, with regular access to email. Participants
could not have been involved in another consumer use study in the
previous 2 months; if they were using other anti-hair loss products,
they agreed to stop using the products one month prior to the start of
this study. Participants also agreed to complete study questionnaires
on Week 4 (ending 14 April 2024), Week 8, (12 May 2024), and Week
12 (9 June 2024).
Participants who were using treatment for severe conditions such
as cancer, diabetes, or Parkinson’s disease, or who were on morphine
or had hair transplants were excluded. Additional exclusion criteria
were lack of participant availability on study dates due to holidays,
illness, or travel; planned vacations during the study period; pregnancy,
likelihood of pregnancy, or breastfeeding; known scalp sensitivities;
issues with taste or smell; ongoing medical or dermatologic treatment;
history of allergies to cosmetic products; simultaneous participation
in another clinical study; and/or employment in a profession related
to cosmetics, hygiene, or hair care, including close household or
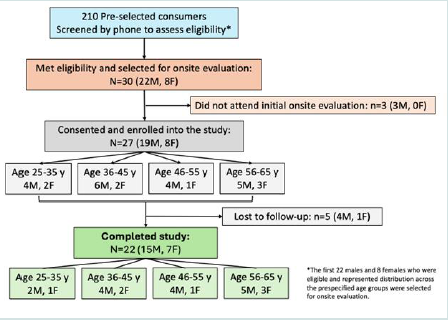
family ties to these industries. [Figure 1] shows the flowchart for study
participants screened, enrolled, and completing the USPlus DERM
Topical consumer use study.
At the first study visit (Week 0/baseline), participants underwent
dermatologic assessment to evaluate their stage of hair loss, using
established classification systems for male and female pattern
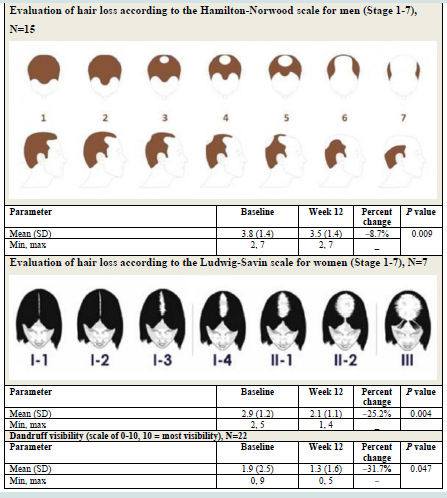
baldness. Hair loss scoring was done using the Hamilton-Norwood
scale for men (Stage I [minimal or no hair loss] to Stage VII [only thin
band of hair remaining], with each stage corresponding respectively
to 1-7 points) and the Ludwig-Savin scale for women (Stage I-1 to
I-4 [minimal thinning], II-1 to II-2 [moderate thinning], and Stage
III [severe thinning], with each stage corresponding respectively to
1-7 points) [32]. Dermatologic evaluations of dandruff visibility, skin
phototype (Fitzpatrick scale, Types I [maximum sun sensitivity] to VI
[lowest sun sensitivity]), and scalp health were also made at baseline.
The dermatologist examined participants’ scalps for any
discomfort or physical signs, and baseline photographs were taken.
Also at this visit, participants were provided with blinded 2% USPlus
DERM Topical serum in plain glass bottles labeled “growth serum”
and with product lot number information.
Participants were instructed to maintain their usual shampoo and
hair care regimen without modification throughout the study, and to
apply the product 4 times weekly (3 applications on clean, wet hair,
and 1 application on dry hair) over the next 12 weeks. Prior to product
application, participants were directed to shake the bottle. Next, they
were instructed to apply two pipettes of serum to the roots and scalp,
massage it in for several minutes, and leave the product on without
rinsing. A 3-item questionnaire, administered at Weeks 4, 8, and 12,
asked participants to confirm whether they were adherent to the use
of the product and the frequency of use as directed, and to document
any reasons for treatment non-adherence over the preceding 4 weeks.
A final dermatological assessment occurred at Week 12, and
study-end photographs were taken. The primary outcome was in
hair loss and overall scalp condition following use of USPlus DERM
Topical 2% serum for 12 weeks. Secondary outcomes were assessed
using participant-reported data obtained at Weeks 4, 8, and 12
from a 15-question, 4-point semantic scale (1=agree, 2=somewhat
agree, 3=somewhat disagree, and 4=disagree) that evaluated overall
improvements in hair loss and hair density, and hair characteristics
such as body, health, suppleness, softness, strength, shine, and
thickness. Participants also appraised the product’s impact on
hair loss, density, regrowth, scalp stimulation, and the visibility of
affected areas, and provided their opinion regarding the impact of
the product on daily hair loss and hair loss when styling. In addition
to the semantic scale questionnaire, study participants completed 3
numeric rank questions evaluating general hair loss (0=no hair loss at
all to 10=severe hair loss), hair loss when styling (0=no hair loss at all
to 10=severe hair loss), and hair density (0=very low hair density to
10=very high hair density) after 4, 8 and 12 weeks of USPlus DERM
Topical use. Participants were also asked a yes/no question regarding
whether they noticed an improvement in hair loss or density, and, if
applicable, at which study Week they observed this change. Last, at
Week 12, participants completed a product satisfaction questionnaire
Statistical Analysis:
Descriptive statistics were used to summarize data, including
means, standard deviations (SD), counts, and percentages. For
dermatologist-assessed hair loss and appearance of dandruff, and
for participant assessments using numeric ranking (0-10), the
Student’s t-test was applied, comparing responses at Week 4, 8, and
12 vs baseline. For participant assessments using the 4-point semantic
scale, a Z-test was applied, comparing the proportion of participants
who “agreed” or “somewhat agreed” to participants who “disagreed”
or “somewhat disagreed” at each discrete time point (Weeks 4, 8,
and 12). The Z-test was also applied to compare the proportion of
participants who did vs did not notice an improvement in hair loss
or density each week. The threshold for statistical significance was set
at 0.05. Analyses were performed using Microsoft Excel (Redwood,
WA, USA) and Modalisa (Paris, France).Results
Participant Characteristics:
Of the pre-selected consumers, 30 (22 men, 8 women) met
eligibility criteria and were subsequently recruited; of these, 27
attended the baseline visit and consented to be in the study. A total
of 22 (73.3%) participants completed the study and were included in
the analysis (n=5 lost to follow-up; Figure 1). Treatment compliance
was high; all participants reported using the LSESr serum as directed
through the end of the study, with the exception of 3 participants
(2 ran out of serum a few days before study end; 1 forgot to use the
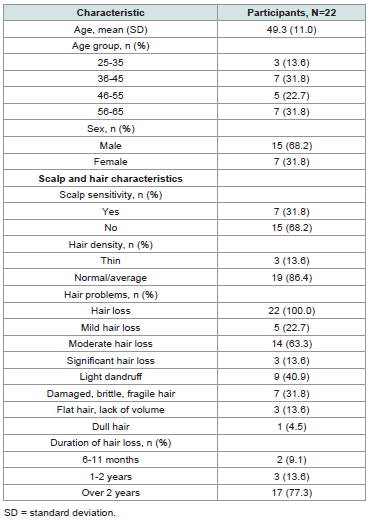
serum during Week 11).[Table 1] and (Table S2) show baseline participant demographic
and hair/scalp characteristics. The study population consisted of 15
[68.2%] males and 7 [31.8%] females, with mean (SD) age at study
entry of 49.3 (11.0) years (range, 25-65). All participants reported
hair loss of a mild (5 [22.7%]), moderate (14 [63.3%]), or significant
(3 [13.6%]) level, with 77.3% of participants (17) experiencing hair
loss for longer than 2 years. With respect to hair characteristics,
participants had normal (8 [36.4%]) or normal to dry hair (6 [27.3%])
that was straight (12 [54.5%]) or wavy (7 [31.8%]) and very short
(5 [22.7%]) or short (8 [36.4%]), with normal/average density (19
[86.4%]). Most participants had either skin phototype III (7 [31.8%])
or IV (9 [40.9%]) and a dry (8 [36.4%]) or normal (10 [45.4%]) scalp;
7 (31.8%) reported scalp sensitivity. In addition to hair loss, some
participants reported hair problems such as dandruff (9 [40.9%]) and
damaged, brittle, or fragile hair (7 [31.8%]).
Outcomes:
In this pilot clinical trial, mean change in participanthair loss and
overall scalp condition following USPlus DERM Topical treatment
are shown in [Table 2] (Table S3) shows individual scores). FromWeek 0 to Week 12, dermatologist-assessed mean (SD) hair loss
for men using improved significantly, decreasing by 8.7%, from 3.8
(1.4) to 3.5 (1.4), according to Hamilton-Norwood scale (P=0.009).
Similarly, for women, dermatologist-assessed mean (SD) hair loss
improved significantly, decreasing by 25.2%, from 2.9 (1.2) to 2.1
(1.1), according to the Ludwig-Savin scale (P=0.004). Dandruff
visibility also improved significantly, decreasing by 31.7% from 1.9
(2.5) to 1.3 (1.6) (P=0.047). Photographic evidence confirming hair
loss improvements is shown in (Figure S2).
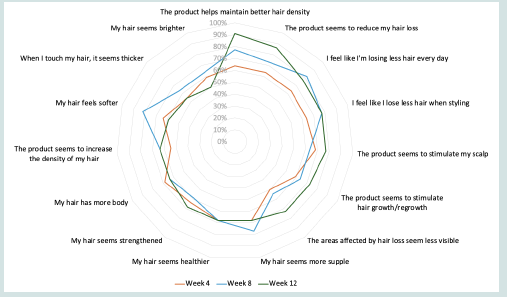
[Figure 2] and (Table S4) show responses to the 15-item
questionnaire administered at Weeks 4, 8, and 12 to evaluate
participants’ perceptions of how treatment affected their hair and
scalp. By 4 weeks of serum use, 13 (59.1%) of participants agreed/
somewhat agreed that the product seemed to stimulate hair growth/
regrowth, and 14 (63.6%) agreed/somewhat agreed that the product
helped maintain better hair density, but the difference between these
proportions and the proportion of participants who disagreed/
somewhat disagreed was not significant. By Weeks 8 and 12, this
proportional difference was significant, with 17 (77.3%) (P<0.05)
and 20 (90.9%) (P<0.001) agreeing/somewhat agreeing, respectively.
By Weeks 8 and 12, participant-reported findings that the product
reduced hair loss were significant, with 16 (72.7%) (P<0.05) agreeing/
somewhat agreeing at Week 8 and 19 (86.4%) (P<0.01) agreeing
Figure 2: Radar Chart of Participant-Reported Efficacy at Weeks 4, 8, and 12 of USPlus DERM Use.
Percentages reflect the frequency of participants (N=22) who “agree” or “somewhat agree” with the given statements at each time point.
somewhat agreeing at Week 12. Also, by Week 12, 16 (72.7%) (P<0.05)
of participants agreed/somewhat agreed that the areas affected by hair
loss seemed less visible, and that the product seemed to stimulate hair
growth/regrowth. Last, 17 (77.3%) of participants agreed/somewhat
agreed that the product seemed to stimulate their scalp (P<0.05)
and indicated that they felt they were losing less hair when styling
(P<0.05) and every day (P<0.05).
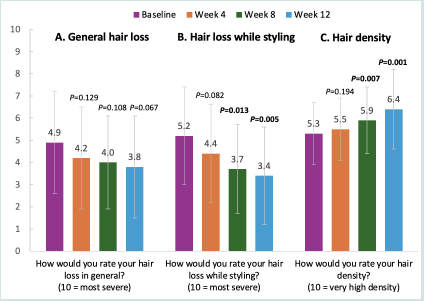
[Figure 3] shows participant rankings (ranging from 0 to 10) of
general hair loss, hair loss with styling, and hair density at Week 0 and
after Weeks 4, 8, and 12 of using USPlus DERM Topical. At baseline,
participants rated their hair loss in general as a mean 4.9 (2.3) out of
10; at Week 12, hair loss in general was reduced by 22.4%, to 3.8 (2.3)
(P=0.067). Participants rated their baseline hair loss while styling as
a mean (SD) 5.2 (2.2) out of 10; the rating was reduced significantly
by 34.2% (to 3.4 [2.2]) at Week 12 (P=0.005). Likewise, at baseline,
participants ranked their hair density as a mean (SD) 5.3 (1.4) out
of 10; at Week 12, this score improved significantly, by 19.5%, to 6.4
(1.8) (P=0.001).
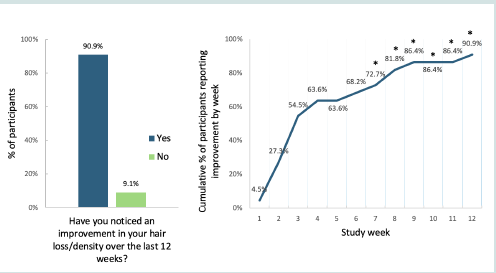
[Figure 4] and (Table S5) show the study timeline, by week; this
illustrates if and when participants observed benefits in hair loss and
density. Progress was rapid, with 14 (63.6%) noticing improvement
by the end of Week 4 of USPlus DERM Topical use. The difference
in the proportion of patients who did vs did not notice improvement
was statistically significant by the end of Week 7 (16 [72.7%]; P<0.05),
and increased to 18 (81.8%; P<0.01) by the end of Week 8, and 20
(90.9%; P<0.001) by the end of the study. Only 2 (9.1%) participants
reported noticing no improvements over the study period.
(Figure S3) shows participant-reported product satisfaction,
evaluated at the end of the study. Participants ranked their
satisfaction with the product as a mean 7.7 out of 10, with over one half
(13 [59.1%]) ranking their satisfaction as ≥8 out of 10. Nearly
all participants (20 [90.9%]) indicated they would like to continue
using the product, and the majority indicated they would “certainly”
or “probably” purchase the product (16 [72.7%]) and recommend the
product to someone experiencing hair loss problems (17 [77.3%]).
(Table S6) shows participant evaluation of product characteristics
and sensation. At Week 12, all participants (22 [100.0%]) “agreed” or
“somewhat agreed” that the product was easy to apply and pleasant
to apply on wet or dry hair, and nearly all (20 [90.9%]) “agreed” or
“somewhat agreed” that the texture and fragrance of the product
was pleasant, and that the product was suitable for their hair type.
The differences in the proportions of patients who agreed/somewhat
agreed with these statements was statistically significant compared to
the proportion of patients who disagreed/somewhat disagreed.
Overall, per dermatologist-assessment at Week 12, the product
was well tolerated. One moderate case of erythema was reported
(unrelated to product use). In addition, there was 1 case of severe
burning/itching that occurred just after product application,
but spontaneously regressed and did not recur with subsequent
applications; and 1 case of moderate itching that was determined to
be unrelated to the product.
Discussion
USPlus DERM Topical 2% hair growth serum was formulated
with a new, proprietary LSESr ingredient containing concentrated
amounts of the most bioactive FFAs [25,28,30] found to be
important for hair structure and hair characteristics [23,24,26]
and to play an important role in regulation of the hair growth cycle
[24,27]. The ingredient used in the hair growth serum, USPlus
DERM, is a standardized quality saw palmetto extract that meets the
United States Pharmacopeia Monograph for potency, purity, and
authenticity. This 3-month, observational, consumer-use pilot study
of USPlus DERM Topical 2% serum found significant improvements
in hair loss reduction and hair density based on both dermatologist assessed
and participant-reported outcomes. Study participants were
age-distributed (25-65 years), had a range of hair and skin types, and
largely had a duration of hair loss ≥2 years. At study end (12 weeks),
Figure 3:Participant-Reported (N=22) Ranking of A) General Hair Loss, B) Hair Loss with Styling, and C) Hair Density from Baseline to Week 12.Participants were asked to rank these items on a numeric scale of 0 to 10. For 3A, numeric ranking of hair loss, less hair loss is indicated by a decrease in score. For 3B, numeric ranking of hair loss when styling, less hair loss is indicated by a decrease in score. For 3C, numeric ranking of hair density, an increase
in score indicates improvement for hair density. All P values are vs baseline.
Figure 4: Participant-Reported (N=22) Improvement in Hair Loss/Density from Baseline to Week 12. An asterisk indicates a statistically significant difference in the proportion of participants who did vs did not notice improvement
dermatologist-assessed male PHL decreased by 8.7%, and female PHL
by 25.2%, and a 31.7% reduction in visible dandruff was also seen. The
majority of participants (90.9%) noticed less hair loss and improved
hair density, with over one-fifth (27.2%) observing changes within 2
weeks of product use, and two-thirds (63.6%) within 4 weeks of use.
By 8 weeks, participant assessment of general hair loss and hair density
improved by 18.4% and 11.3% (P=0.129 and P=0.007, respectively),
and hair loss when styling decreased by 28.9% (P=0.013). At study
end, participant-assessed hair loss reduction in general compared to
baseline decreased by 22.4%, hair density improved by 19.5%, and
participants reported a 34.2% reduction in hair loss when styling hair.
Additionally, three-quarters of participants reported that the areas
affected by hair loss seemed less visible (72.7%), that they felt they
were losing less hair every day (77.3%), and that the product seemed
to stimulate hair growth/regrowth (72.7%).
Furthermore, product satisfaction was high (7.7 out of 10),
with 90.9% of participants indicating interest in continued use of
the product after the study end, and 72.7% indicating interest in
purchasing the product, regardless of the cost. Compliance with
serum application/use was also high, and the product was well tolerated,
with no side effects attributable to its use. Given these
results from this pilot clinical trial, the combined efficacy, tolerability,
and acceptability of USPlus DERM Topical makes this product a
feasible option for addressing hair loss and hair regrowth [4].
Hair loss, including pattern hair loss, can negatively affect an
individual’s self-image, with research confirming that PHL can cause
emotional and psychological distress due to worry, embarrassment,
shame, frustration, and/or depression. In turn, this can negatively
affect health-related quality of life (HRQoL). Likewise, having
access to PHL treatment improves HRQoL [33]. Many individuals
with PHL are seeking natural treatment options that offer effective
results with a lower risk of side effects (adverse events associated with
PHL prescription therapy includes dry scalp, skin irritations and
reddening, headache, and sexual and ejaculatory dysfunction) [6].
The current study gives insights into the onset of action of topical
application of a LSESr serum on hair loss, as well as its impact on
hair characteristics and other important conditions such as dandruff.
These clinical results are supported by prior research, spanning more
than two decades, showing that saw palmetto extracts, administered
as oral therapy [10, 11, 14-16]or in topical formulations [9, 12,
13, 17] can play a key mechanistic role in preventing hair loss and
promoting hair regrowth. Specific to oral therapy, Rossi et al (2012)
studied 100 men with androgenetic alopecia to determine the 2-year
comparative effectiveness of LSESr monotherapy and the 5αR
therapeutic finasteride. LSESr monotherapy use resulted in a 38%
improvement in hair regrowth, compared to a 68% improvement
in the finasteride group [16]. Ablon and Kogan (2018) conducted a
6-month randomized clinical study of women with self-perceived
hair thinning, receiving either an oral LSESr combination hair growth
supplement (n=24) or a placebo (n=12). By day 180, participants
using the LSESr combination product had significantly improved
hair growth and overall hair quality as assessed by an investigator, as
well as enhanced self-perception of hair growth, volume, thickness,
and overall hair health [10]. A subsequent 6-month randomized trial
conducted by Ablon and Kogan (2021) to evaluate the same oral
LSESr combination product randomized menopausal women with
thinning hair to receive active treatment (n=40) or placebo (n=30).
Compared to placebo, participants using the LSESr product showed
progressive and significant decreases in hair shedding by day 180
(mean reduction, 32%), as well as significant increases in terminal,
vellus, and total hair counts [11]. As part of a randomized, controlled
4-arm study of adult men and women with androgenetic alopecia
(N=80), Sudeep et al (2023) evaluated a nonconventional saw
palmetto ingredient, delivered as both an oral and topical product
and evaluated for 16 weeks. By study end, the oral product was shown
to reduce hair shedding by up to 29% and to increase hair density by
5.2%. Additionally, participants taking the oral saw palmetto product
showed significant reductions in DHT levels compared to placebo
(P<0.007) [17]. Across these studies, the oral LSESr products were
well-tolerated, with no [10,16,17] or only minor [11] adverse events
In addition to saw palmetto oral supplements, research evaluating
LSESr for topical application has included the use of lotions and
serums, in combination with other active ingredients [9,12,13]
or as a single active ingredient [17]. LSESr topical combination
products have shown improvements in hair density and hair mass
[9,12-14]. Morganti et al (1998) evaluated the topical application of
a combination LSESr lotion over 50 weeks in men and women with
androgenetic alopecia (n=24 in each group). The LSESr combination
lotion increased hair mass and hair number starting at week 10; by
study end, the LSESr combination lotion increased hair mass by 30%
and increased hair number by 27%[13]. Wessagowit et al (2015)
conducted an open-label study of 50 men with androgenetic alopecia
using a LSESr topical combination serum applied for 4 weeks, followed
by a LSESr combination lotion for 20 weeks. This regimen increased
terminal hair count compared to baseline at 12 and 24 weeks, decreased
medium-sized and vellus counts at 24 weeks, and changed median
androgenetic alopecia stage from 4 to 3 at 24 weeks [9].In a doubleblind
randomized study, Masoud et al (2020) evaluated a topical
LSESr combination product with rosemary oil plus 5% minoxidil
(n=12) versus 5% minoxidil (n=12) in men with mild to moderate
androgenetic alopecia and found that use of the LSESr-containing
product significantly improved hair diameter compared to minoxidil
alone, and significantly improved hair density compared to baseline.
Study participants who added this LSESr combination to minoxidil
noted increased hair growth, hair loss reduction, and improved hair
appearance [12].As noted previously, Sudeep et al (2023) conducted a
4-arm randomized controlled study in participants with androgenetic
alopecia to evaluate a nonconventional saw palmetto ingredient; in
this analysis, the topical product was formulated in a concentrated
20% lotion, applied for 16 weeks in adult men and women. By study
end, the lotion was shown to reduce hair shedding by up to 22.2% and
to increase hair density by 7.6% [17]. All of these LSESr-containing
topical products and the nonconventional saw palmetto topical lotion
had a positive effect on hair regrowth and scalp appearance and were
associated with only minor side effects. Treated individuals report
high rates of satisfaction [9,12,13,17].
Over 12 weeks, the use of a 2% serum of USPlus DERM led to
dermatologically assessed improvement in hair loss in both men and
women. Topical application of the LSESr had a significant impact on
hair density and led to a significant reductions in hair loss with styling.
The use of this proprietary LSESr with concentrated bioactive FFAs
also improved hair characteristics and led to reduction in dandruff, as
reported by the study participants and on dermatologic assessment,
respectively.
Conclusion
This blinded pilot consumer use trial showed that USPlus DERM,
applied topically as a 2% serum (USPlus DERM Topical), improved
hair characteristics, supported hair structure and hair density, and
addressed hair loss and hair regrowth in participants with a range
of skin and hair types, including many individuals who had been
experiencing hair loss for 2 years or longer. At 12 weeks, 90.9% of study
participants indicated an improvement in hair density and less hair
loss. The product was well-tolerated, and its sensory characteristics
were well-accepted. These results indicate that USPlus DERM with
concentrated bioactive FFAs is an effective LSESr for men and
women looking for a natural solution to address hair loss, improve
hair density, and support healthy hair growth and characteristics.
Acknowledgements
Caitlin Rothermel, MPH (MedLitera) provided editorial
assistance. The authors would like to thank Jean-Nicolas Chabrel
of VENDEIS for coordinating the development of the 2% USPlus
DERM Topical serum and clinical study with SYRES Sensory &
ConsumerResearch. The 2% hair growth serum formulation was
developed by Catherine Taurin, Capillaires Conseils. The authors
would also like to thank the volunteers who participated in the
consumer use test. The study was funded by Valensa International.
Conflicts of Interest:
W. Stephen Hill and Margaret H. Dohnalek are employees
of Valensa International. Catherine Carletto is a consultant to
VENDEIS, which distributes the active ingredient for the USPlus
DERM Topical formulation.