Journal of Cancer Sciences
Download PDF
Review Article
Artificial Intelligence in Radiation Oncology: Applications and Future Direction
Wang T1,3, Goel HL1-3, Ding L1, Bradford C1, Khalifeh A1, Liu F1, Fan Y1, Kuo I-L1, Larosa S1, Yancey J1, Jones G1, Harris D1, Bishop-Jodoin M1, Smith K1, Iandoli M1, Laurie F1, Goel S2 and FitzGerald TJ1*
1Department of Radiation Oncology, University of Massachusetts Chan Medical
School, Worcester, MA, USA
2Department of Molecular, Cell and Cancer Biology, University of Massachusetts Chan Medical School, Worcester, MA, USA
3Co-first Authors
2Department of Molecular, Cell and Cancer Biology, University of Massachusetts Chan Medical School, Worcester, MA, USA
3Co-first Authors
*Address for Correspondence:Thomas J FitzGerald, Department of Radiation Oncology, University of
Massachusetts Chan Medical School, Worcester, MA, USA E-mail Id: TJ.FitzGerald@umassmemorial.org
Submission:28 February, 2024
Accepted:27 March, 2024
Published:02 April, 2024
Copyright:© 2024 Wang T, et al. This is an open access article
distributed under the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original work is properly cited.
Abstract
The practice of radiation oncology is changing at a rapid
pace. As models of care and department reimbursement change,
departments are evaluating an increasing number of patients with
complex medical backgrounds which must be acknowledged as
increasing difficult treatment plans are developed. With altered
fractionation strategies, compressed fractionation treatment delivery,
and highly sophisticated radiation therapy treatment technology,
radiation therapy has evolved into a highly rigorous discipline applying
multiple overlapping image datasets to define targets of interest with
sophisticated image guidance tools to ensure accuracy of therapy.
Integrated with biomarkers associated with treatment response and
therapeutic resistance, radiation therapy can be delivered to tumor
with altered doses to image-guided subsets of disease. The datasets
now required to generate radiation oncology treatment plans are
becoming increasingly large and complex. Ultimately, artificial
intelligence models will mature to ensure accuracy and consistency to
department workflows and therapeutic decisions including contouring
and treatment planning. Artificial intelligence can and will be applied
to all elements of daily patient care as well as clinical translational
research. In this paper, we explore how multiple components of
artificial intelligence will support the radiation oncology work force
and therapy-guided clinical trials moving forward.
Introduction
Artificial intelligence (AI) is described as the science and
engineering of making intelligent machines and computer programs.
[1] The field of AI combines increasing sophistication of computer
science and the incorporation and integration of datasets to enable
problem solving and translational research through computer driven
operations. In the clinical trial environment, investigators are
expected to anticipate, during the clinical trial design, events that are
predictable and how they should be managed if a patient is treated in
a protocol compliant manner.[2] However, in the clinical trial setting
or within daily department function, it is often an event or events,
good or bad, that are unanticipated which require more detailed
understanding of the data to determine root cause of the event and
resolve problems or inconsistency in management. The data need to
be reviewed and studied to determine if, whether or not, information
in the patient history, genomic/proteomic biomarker assessment, or
medical treatment de novo influenced the unanticipated outcome and
whether or not the event could have been prevented with improved
knowledge derived from AI models generated from established
databases of patients treated in a similar manner. AI has two subfields:
machine learning and deep learning. Although the terms are often
applied in an interchangeable manner, there are nuanced differences.
Both are sub-fields of AI and deep learning is a sub-field of machine
learning. Machine learning is a human-driven process of pattern
recognition designed to automate predictable and reproducible
functions at a primary level. Deep learning is comprised of neural
networks and the term “deep” refers to a neural network of more than
three layers with a layer between input and output. Deep learning
automates the process of features extraction, thus decreasing the
human intervention required for machine learning and training. [3]
Both processes leverage labeled datasets to inform and promote the
computer algorithm. Machine learning requires human intervention,
thus deep learning becomes a scalable form of machine learning. Both
require structure with deep learning potentially providing an answer to
a question that human intervention may or may not see. Most current
AI programs function at this level. The next step in the development
of AI is in the development of natural language processing including
various data types including but not limited to molecules, images,
and the grammar of software code. In this future capacity, AI may
have the capability of identifying and anticipating what we do
not yet see. Recent development of Chat Generative Pre-Trained
Transformer (ChatGPT) and sibling models including Instruct GPT
rely less on human intervention for activity but interpret words to
generate a response.[4] The human training in ChatGPT is extensive
and influences both input of information and response generated
for the output of information, however it generates responses from
training by calling upon multiple layers of input including context
and tone, therefore generating more sophisticated responses than
current engines managed exclusively by human input and output.
This is referred to as a generative AI model. These datasets are trained
on vast amount of information including the internet, websites news
articles, scientific articles and more.[5]Generative AI also is provided
periodic feedback for process improvements. These tools are not yet
integrated into AI models in radiation oncology; however, it will not
be long before generative models are integrated into our workflow
providing checks and balances on our work and potentially serving
as a mechanism for department and clinical trial quality assurance.
Radiation oncology heavily relies on computer data processing,
making it a branch of healthcare that can greatly benefit from
advancements in AI. Deep learning, or neural networks, is designed
to emulate the human brain, and continuous advancements have
the potential to surpass human intelligence. AI validation involves
evaluating trained models on testing datasets, providing insights into
the model’s overall effectiveness and applicability. AI models will
have influence in all aspects of radiation oncology. Today, nearly
all AI programs in our discipline function at an early iterative level
recognizing patterns in information and taught to recognize patterns
and extract features based on what has been defined by specific human
interactions. [6] By modern current standards the components are
nascent however important to current daily department function. In
the following sections, we will review current processes influenced by
programs in AI and how they may be applied in the clinic today and
in the future.
Administration and Regulatory Functions:
The process of consult request in radiation oncology and obtaining
information relevant for processing the consult have multiple
predictable and reproducible steps which lend well to support from
AI. AI programs currently exist and are applied routinely for customer
satisfaction, and these can be re-purposed for chart preparation
and consult.[7] The AI models at this level will permit departments
and institutions to track demographics for referral patterns and
additional information for growth of patient volume. The models
will review information in the electronic medical record for chart
completeness including regulatory compliance. This is important
as electronic medical records including Epic do not have a module
for radiation oncology. This is due to proprietary software imbedded
within the electronic record in radiation oncology which direct and
validate simulation, quality assurance computational components,
and daily treatment. Because the software directly affects the function
of the linear accelerator and quality assurance processes, accelerator
companies such as Varian have not made the proprietary software
available to electronic medical record companies such as Epic.[8]
Therefore, institutions need to be creative in the development of
interfaces to achieve the objectives for regulatory compliance for
department processes. AI programs can provide cross reference within
each record to facilitate transfer of objects between the systems. For
example, Epic uses a program called Beacon for medical oncology.
When radiation oncology departments are accredited for practice by
the American College of Radiology (ACR) or the American Society for
Radiation Oncology Accreditation Program for Excellence®(ASTROAPEx),
reviewers either onsite or using remote tools will only review
objects from one electronic medical record. By default, the review
must be from the electronic record in radiation oncology as it houses
daily treatment quality assurance, daily treatment images, and
computational analytics required for daily patient care. Interfaces,
however, have imperfections and will both move unnecessary notes
into the radiation treatment record and not move necessary notes
[9]. This can often require human oversite to be certain the correct
notes are in place at the time of regulatory review. AI models can
align the correct note to the correct day which can in turn secure
regulatory review and improve compliance to billing objects. Once
key words can be incorporated into the AI models, the models can be
re-purposed for department quality assurance regulatory compliance
including insurance authorization. The standards for accreditation
are increasing. Physician history and physical examinations and
documentation are under significant scrutiny for regulatory and
insurance compliance. Elements for a Level 5 consult can be reviewed
by models for compliance. AI models can ensure key elements are
addressed in a templated format to ensure compliance. The elements
in a radiation oncology treatment chart including physics, treatment
planning, simulation notes, on treatment visits, and completion/
follow up summaries. AI models can identify gaps in documentation
to improve compliance. Speech recognition technologies are a
version of AI and coupled with templates, facilitate both compliance
and throughput for document completion.[10]These and other tools will prove invaluable to practice
management teams as trends in volume can be managed in real time
with direct feedback to providers and referral sites. The tools will make
practices more efficient and increase vehicles and opportunities for
communication between practices in order to facilitate department
growth.
Patient Management:
Modern radiation oncology requires metrics and pathways
for patient management. Once a patient has agreed to therapy, the
radiation oncologist needs to write a therapy directive for treatment
and populate the record for directives for image guidance, dose
volume constraints, and insurance approval. Many disease sites
in radiation oncology have predictable metrics assuming normal
functional anatomy. For example, prostate teletherapy with intensity
modulation has dose volume constraints which often can be applied
across a uniform patient population.[11] Therefore, dose to bladder,
rectal, and small bowel volumes can often be applied in a uniform
manner and AI models can be used to auto-populate documents at
the discretion of the physician. These can be revised as needed for
patient-specific issues including history of ulcerative colitis and
other pre-existing medical comorbidities as needed by physician specific
interaction for dose volume adjustment for metrics.
These models can be developed for all common disease sites with
predictable structure and functional status including radiosurgery,
brachytherapy, and all advanced technology radiation therapy forms
of teletherapy. Often insurance directives request comparison of
intensity modulation driven plans and plans developed with three
dimensional technologies which can be a time burden on physics and
dosimetry staff. AI models can generate these plans for comparison
and review by insurance companies, saving both time and resources
for providers of care allowing planning teams to dedicate more time
to traditional work.[12,13] This has significant relevance to modern
practice as even departments dedicated to advanced technology
treatment delivery are often witnessing a selective general decrease
in revenue per patient as compressed fractionation becomes a more
common practice. Many departments are witnessing new patient
growth which is required for budget however even these departments
have a decrease in revenue per patient due in part to a decrease in
the number of treatments despite an increasing number of patients.
To maintain similar revenue and maintain cost despite increasing
numbers of new patients, departments will need to apply AI tools to
facilitate throughput and maintain quality with a similar number of
staff. [14] This provides an economy of scale as AI models can direct
and complete tasks with more predictable structure and endpoints
and staff can monitor the AI models and devote time to tasks that are
complex, less predictable, and mandate direct human intervention.
[15]Segmentation, or contouring, is a crucial and essential step
in radiation oncology. Accurate segmentation is vital because
it determines the effectiveness of the treatment. The successful
implementation of AI-based auto-segmentation was also
demonstrated in prostate radiotherapy by Cha et al. [16]. Combining
AI-based segmentation with the expertise of radiation oncologists can
further enhance treatment efficacy and improve outcomes. Manual
segmentation is very difficult in Neuroendocrine tumors, Santilli et
al. successfully used nnU-net pipeline for automatic segmentation
of tumor images, generating accurate segmentation masks.[17]
Kazemimoghadam et al. utilized a deep-learning model on CT images
of breast cancer patients and achieved promising results for accurate
delineation of clinical target volume (CTV) [18]. An FDA-approved
deep learning algorithm (VBrain) showed promising results for brain
metastases segmentation.[19]
In radiation oncology, the likely most influential area AI models
will impact in the next five years will be in image integration and
radiation therapy treatment planning. Historically radiation therapy
plans were developed using two-dimensional tools using fluoroscopy
as the primary imaging vehicle [20]. Radiation dose was defined at
the isocenter of the target and calculated to an isodose line. Today,
radiation therapy is calculated to normal tissue and tumor volumes
are contoured on three- and four-dimensional target volumes
with objects superimposed of digital platforms. Dose is made
uniform through the use of intensity modulation made facile by
the presence of multi-leaf collimators housed within the gantry of
the linear accelerator. However, the critical step for the radiation
oncologist in the planning process is to contour the target volumes
of interest including normal tissue for conformal avoidance and
tumor targets for therapy. This is a critical component in treatment
planning for radiation therapy. Today, the data sets for contouring
targets are complex and often require multiple integrated datasets
to complete contouring for patient care [21]. Radiation oncology
today is an exercise in applied imaging. While the quality assurance
of computational analytics and therapy delivery require significant
quality assurance, the uncorrectable aspect to a radiation oncology
treatment plan is the accuracy of physician contours.[22] If tumor is
under contoured or disease over contoured into critical normal tissue,
patient outcomes can be negatively influenced by disease progression
or normal tissue injury. This is how radiation oncology has changed
over the past several decades. Feasibility of auto-contouring of
neovascular structure in prostate cancer patients was recently shown.
[23]
CT and volumetric treatment planning have become the standard
of care for patient management including clinical trials involving
radiation therapy [24]. Each step in the process of image acquisition
and contouring can be facilitated and assessed for quality assurance
by models for AI. The radiation oncologist will write a simulation
directive describing the goals and objectives of the simulation process
and images required for fusion into radiation oncology planning
images for contour definition. At this point images are obtained per
physician directive at slice thickness commensurate with the objectives
of the simulation. Historically, the work scope of the radiation
oncologist often concluded with the completion of the simulation.
Today, the work scope only begins with the end of the simulation
hour. After an initial isocenter is placed, as a point of reference to
accurately reproduce daily therapy, physics planning teams and the
involved radiation oncologist develop a strategy for next steps in
management. In an uncomplicated situation, often the objective can
be directed to the task pad of the radiation oncologist for contour of
targets. In multiple disease areas, however, fusion of additional images
is essential for contour as many targets cannot be well visualized
on radiation oncology planning CT studies, therefore targets need
additional fusion of datasets to optimize contouring for patient care.
Central nervous system management, especially targets for primary
disease and for stereotactic radiation therapy require fusion of MRI
objects to complete contouring in an accurate manner as often targets
cannot be visualized on CT, even including contrast during the
simulation. Future protocols for disease in the central nervous system
will include multiple magnetic image datasets as, interestingly, each
provides a different view of what could be tumor. For example, a
modern protocol currently evaluates spectroscopy, fluid attenuated
inversion recovery (FLAIR), and T1 signal with contrast to define
targets with each area receiving a differential radiation dose using
dose painting for treatment execution. Investigators are currently
evaluating the use of positron emission amino acid imaging as a study
to define the area of DNA synthesis within the tumor target as an
additional area for therapy with augmented fractionation directed to
the target volume defined by additional imaging tools. For contouring
multiple datasets, registration and segmentation of images and targets
requires precision and accuracy. AI models can both facilitate the
processing of integrating images for target definition and ensure the
accuracy of the integration of the datasets [25]. Multiple disease areas
will be favorably influenced by AI models. Head and neck therapy is
becoming increasingly significant for several reasons. The prevalence
of this disease is rising, particularly among patients with viral etiology.
Within this group, some patients experience positive outcomes
and may benefit from tailored treatment adjustments. In a similar
manner, investigators are evaluating the merits of volume titration
in this disease to further promote improvement in normal tissue
outcomes understanding the potential risk of recurrence in treating
decreased volumes of both regional and primary target regions.
Surgery is increasing in utility for this disease as it is often curative
without additional therapy. In this circumstance, radiation therapy
can be deferred until there is another event. In the event radiation
therapy is recommended on a post operative basis, AI models can be
used to define areas of risk at/beyond the surgical resection margin
as well as additional lymph nodes at risk including sites of extra
capsular extension [26]. This would further ensure targets at high risk
will be incorporated into the therapy field and limit dose to targets of
unintended consequence including the mandible, parotid glands, and
spinal cord. There is increasing interest in titration of target volumes
in head and neck cancer to limit long term sequelae of management.
AI models have demonstrated potential in head and neck cancer
[27]and hypopharyngeal cancer.[28] Datasets can be developed from
benchmark cases and used as an atlas to compare individual cases to
hone and improve AI models for target volume definition. Patterns
of failure can be incorporated into the models to further refine the
program for protocol management to learn what is reasonable for
volume titration and what may place the patient at a higher risk
of treatment failure. Pulmonary radiation therapy has undergone
significant change in the past two decades. With increasing concern
for toxicity associated with radiation therapy and systemic therapy
including immunotherapy, investigators have placed cardiopulmonary
metrics into protocols which limit risk of a compromised
normal tissue outcome. Tools such as positron emission tomography
serve to further optimize target definition, additional regional nodal
areas of risk and on occasion, additional primary disease. As tumor
targets become better defined, it becomes increasingly difficult to treat
the modern lung cancer patient to elective target volumes as meeting
normal tissue constraints for cardiopulmonary function becomes
increasingly difficult once all areas of metabolically active disease have
been contoured. Motion management adds an additional degree of
difficulty to the radiation therapy planning team as to accommodate
this issue more normal tissue must be incorporated into the therapy
field (ITV) to ensure full tumor target coverage. Recognizing this
issue, AI models can serve to optimize planning in this situation
and can recommend additional strategies of breath hold, adaptive
planning from daily volumetric imaging, and driving dose through
non-functioning sub-segments of pulmonary parenchyma defined on
functional imaging fused into planning imaging.[29] These datasets
will be large and not always intuitive to physics and physician therapy
teams; therefore, AI will be required to identify both the time point
and the strategy for when a meaningful change in target definition is
needed to optimize patient care. Recently, AI was used in identifying
dosimetric predictors of toxicity in cancer patients.[30]
Hepatic radiation therapy is becoming of increasing importance
to the therapy community. The genesis of this disease is multifactorial
in origin, however with a significant worldwide increase in incidence,
therapeutic options need to be developed to improve patient care to this
often-vulnerable patient population. With liver transplant a limited
option for this patient population, local and systemic therapies are
essential and often serve as a bridge to transplant when appropriate.
There are several local therapies available to this patient population
including ablation therapies and systemic radiation therapy, however
each have challenges in treatment of the entire target with unintended
dose delivered to normal tissue targets problematic for infusional and
catheter directed radiotherapy with yttrium-90 (Y-90). Stereotactic
radiosurgery applications have become an important component
to the care of the patient with fractionation patterns directed by
the volume of disease in juxtaposition to the volume of normal
tissue parenchyma. Defining the volume of disease is challenging
and often requires the use of multiple MRI sequences to define the
target volume of interest. Often disease can be less conspicuous to
reviewers, therefore AI models will help both define targets to treat,
assign conformal avoidance strategies to functional parenchyma, and
optimize radiation therapy treatment plans to achieve these important
objectives. This area for radiation therapy has only recently matured as
an important disease site for teletherapy including particles, therefore
many radiation oncologists are less well versed in the challenges of
target definition and treatment execution, therefore most radiation
oncologists will benefit from both the development and utilization
of these models to optimize patient care in this important area for
stereotactic radiation therapy management.[31]
Additional areas of abdominal and pelvic disease are readily
amenable to models for AI. Many abdominal/pelvic targets are
optimally defined on alternate image sets. Mass lesions in the
pancreas and extensions beyond the pancreas are often better defined
on MRI. Fusion of data sets including AI auto contouring tools will
optimize target definition for improvements in radiation oncology
treatment definition and treatment delivery. Efforts to integrate
stereotactic techniques into pancreas radiation therapy have only
seen partial success due to challenges in accurately contouring and
delivering radiation doses precisely across the duodenum.MRI has
supported the development of targets within renal parenchyma for
partial volume therapy in medically appropriate situations. Coupled
with accurate motion management, these targets become important
for radiation oncologists as the patient population is less amenable
to surgical intervention. AI will optimize target definition and
support conformal avoidance to functional parenchyma as partial
volume renal therapy becomes of increasing importance. There are
many situations today where endometrial and cervix brachytherapy
cannot be performed secondary to medical comorbidities. Magnetic
resonance can be used to define high risk areas of residual disease with
radiation therapy treatment plans designed to provide dose painting
strategies to both high and intermediate risk regions to provide care
for the increasing patient population of those who cannot undergo
anesthesia for brachytherapy. The integration of image sets with the
development of teletherapy plans with intensity modulation using
dose painting will be approached and facilitated by programs in AI
as these plans become more commonplace and the therapy strategy
becomes validated. Metabolic images including prostate-specific
membrane antigen studies have provided improved definition of
pelvic and abdominal lymph node regions improving target definition
for regional therapy. As datasets build and can be applied for machine
learning, the process of integrating AI models into daily workflow
processes including planning and quality assurance.[10] Tozuka et al.
recently developed a deep learning model, which showed improved
gamma passing rates prediction.[32]
Conclusion
AI is of increasing importance in radiation oncology for many
practical reasons. Modern therapy requires a significant skill set
among physicians, physics planning teams, and therapy teams.
It is difficult, if not impossible, for an average size department to
recruit individual talent to provide expertise in all areas required for
modern therapy. Reimbursement models in radiation oncology are
also under change. Compressed fractionation strategies in common
disease sites have altered the landscape of the financial infrastructure
for the department. While departments may be evaluating more
new patients as part of therapy management, department treatment
numbers are not commensurate with the increase in new patient
volume. Treatments, however, are more complex including modern
image guidance, however reimbursement is not increasing in parallel
with the complexity of therapy, therefore there is an increasing gap
between the increase requirements for skill and therapy complexity
with reimbursement. Radiation oncology departments are facing
this dilemma and working to develop strategies to address this
dichotomy. AI may help to address this gap by moving more
repetitive department processes into models for AI. [33] A partial list
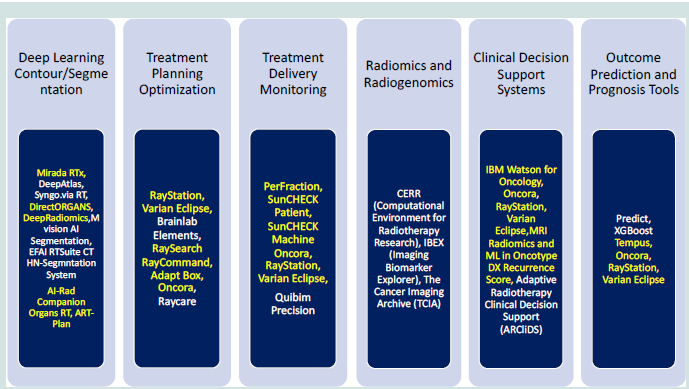
of artificial intelligence programs available for use is seen in (Figure
1). Departments can facilitate this process by building internal
datasets or using databases established by reliable platforms including
the Imaging and Radiation Oncology Core (IROC) and The Cancer
and Imaging Archive (TCIA). The future is bright for applying AI to
all aspects of patient management in radiation oncology. We need to
remain disciplined in our approach and application of these models
in order to optimize both workflow and quality assurance for the
patients we serve.
Conflicts of Interest:The authors have no conflicts of interest to
declare.
Acknowledgements:Dr. FitzGerald’s effort is supported in part by a grant from NIH/NCI - Imaging and Radiation Oncology Core (IROC) U24 CA180803.
Acknowledgements:Dr. FitzGerald’s effort is supported in part by a grant from NIH/NCI - Imaging and Radiation Oncology Core (IROC) U24 CA180803.