Journal of Emergency Medicine & Critical Care
Download PDF
Research Article
A Systematic Review and Meta-Analysis of Intraoperative Goal Directed Fluid and Haemodynamic Therapy in Children and Postoperative Outcome
Kumba C1*, Willems A2, Querciagrossa S1, Harte C1, Blanc T3, De Cock A1, Orliaguet G4 and Melot C5
1Department of Paediatric Anaesthesia and Critical Care, Necker Enfants
Malades University Hospital, France
2Paediatric Intensive Care Unit, Leids Universitair Medisch Centrum,
Netherlands
3Department of Paediatric Digestive and Urologic Surgery, Necker Enfants
Malades University Hospital, France
4Department of Paeditaric and Obstetrical Anesthesia and Critical Care,
Department of Pharamacology and Therapeutic Evaluation in Children and
Pregnant Women, Necker University Hospital, Paris Descartes (Paris V)
University, France
5Emergency Department, Erasme University Hospital, Belgium
*Address for Correspondence: Kumba C, Department of Paediatric Anaesthesia and Critical Care, Necker Enfants Malades University Hospital, 149 Rue De Sèvres, 75015 Paris, France, Tel: 0033144494000; E-mail: claudine.kumba@gmail.com
Submission: 09 April 2019;
Accepted: 02 May 2019;
Published: 06 May 2019
Copyright: © 2019 Kumba C, et al. This is an open access article distributed
under the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original work
is properly cited.
Abstract
Introduction: In adults, studies have shown that when goal
directed fluid and haemodynamic therapy was applied in the
perioperative period, morbi-mortality was reduced. In children the
impact on postoperative outcome of this therapy is not clear.
Objective: To determine the impact of intraoperative goal
directed fluid and haemodynamic therapy on postoperative morbimortality
in children less than 18 years old.
Methods: Systematic review and meta-analysis of randomised and
non randomised studies.
RevMan 5.3 sofware was used for statistic analysis.
Results: 23 studies were included with 3389 children among which
21 trials concerned cardiac surgical children and two concerned non
cardiac patients.
1° In 3290 children in 21 studies included, mortality was significantly
lower in the experimental group (the group with higher above baseline
values of regional oxygen saturation, of mixed central venous oxygen
saturation, and lower lactate levels) (odds ratio=0.03 [0.01, 0.14],
p<0.00001). The quality of evidence (GRADE) was low.
2° In 14 studies with 2347 children included, organ dysfunction was
significantly lower in the experimental group.
(odds ratio = 0.02 [0.00, 0.08], p <0.00001). The quality of evidence
(GRADE) was low.
3° in 8 studies length of hospital stay was significantly lower in the
experimental group (p=0.018). The quality of evidence (GRADE) was
very low.
Conclusions: Intraoperative goal directed fluid and haemodynamic therapy is not developed in children, there are
biomarkers of postoperative adverse outcome in pediatric cardiac surgery.
Keywords
Goal directed fluid; Hemodynamic therapy; Children; Postoperative outcome
Introduction
Background:
In adult surgery, there is evidence that intraoperative Goal
Directed Fluid and Haemodynamic Therapy (GDFHT) improves
postoperative outcome (mortality, morbidity and length of hospital
stay). Several studies have shown that when goal directed fluid and haemodynamic therapy was applied in the perioperative period in high
risk patients, mortality, renal and gastro-intestinal complications and
infections were reduced [1-7]. In children conclusions concerning
this subject are not clear.Why was it important to do this review?:
This review was important to bring some evidence for
pediatric intraoperative management using goal directed fluid and
haemodynamic therapy to improve postoperative outcome and to
develop research in fields where evidence is still unclear and not
developed.How the intervention might work:
The aims of the goal directed fluid and haemodynamic therapy
are to increase end organ blood flow (oxygen delivery) which results
in improved patient outcome. Goal directed fluid therapy and
haemodynamic aim to avoid hypovolemia or hypervolemia which
can compromise end organ perfusion and oxygen delivery.Objectives of this study:
The main objective was to determine whether intraoperative goal
directed fluid and haemodynamic therapy reduced postoperative
mortality and or morbidity in children.Secondary objective was to determine whether intraoperative goal
directed fluid therapy diminished postoperative length of hospital
stay in children.
Methods
This study was registered in Prospero database as [CRD42018103119].
Since this was a systematic review and meta-analysis, ethical
approval from the local ethics committee was not necessary.
Inclusion criteria were randomised and non randomised trials
where intraoperative goal directed fluid and haemodynamic therapy
was applied and compared to standard care in children (patients <18
years old) without any geographic, language or date restrictions.
Intraoperative goal directed fluid and hemodynamic therapy
was defined as interventions where fluids (crystalloids and or
colloids) and or inotropes and or vasoactive drugs were administered
intraoperatively using devices or biomarkers or parameters which
measured goals. Goals were defined as: cardiac output, cardiac
index, oxygen delivery, oxygen delivery index, oxygen consumption,
stroke volume, stroke volume variation, pulse pressure variation,
ScVO2(mixed venous oxygen saturation), lactate levels, oxygen
extraction ratio, aortic velocity-time integral variation, aortic flow
peak velocity, aortic flow peak velocity variation PVI (pleth variation
index), NIRS (near infrared spectroscopy).
Precisely, we designed the interventional or experimental group,
as the group where the intervention was favorable (for instance
higher or normal cardiac index or higher regional oxygen saturation
or higher or normal central venous mixed oxygen saturation ScVO2
or lower and normal values of lactate levels or lower vasoactive
inotropic scores, lower venous to arterial carbon dioxide difference)
or where goal directed fluid and haemodynamic therapy were applied
using haemodynamic devices such as PiCCO (pulse contour cardiac
output), Vigileo, transoesophageal doppler and echocardiography .
The control group was defined as the group where the intervention
was not favorable (for instance lower cardiac index or lower values
of regional oxygen saturation, lower central venous mixed oxygen
saturation ScVO2 or higher lactate levels, higher vasoactive inotropic
scores, higher venous to arterial carbon dioxide difference) or where
standard care was applied. Standard care was defined as situations
where standard parameters were used to monitor haemodynamics
such as mean arterial pressure, perfusion pressure, arterial blood
pressure, central venous pressure. These interventions were applied
intra-operatively (and or in the immediate postoperative period up to
24 h on admission in the PICU (pediatric intensive care unit).
Primary outcome measures were the number of postoperative deaths and number of patients with postoperative complications.
Complications were defined as organ failure or dysfunction or
infections.
Secondary outcome measures were the number of days spent in
hospital
Primary outcomes were postoperative mortality and morbidity
until discharge from hospital. Secondary outcome was Length of
Hospital Stay (LOS).
Search methods for identification of studies:
Titles and abstracts were searched electronically using keywords
between 1 October 2018 and 31 January 2019. Once these were found,
abstracts with relevant content were analysed and complete articles
were searched and screened for further inclusion or exclusion.We searched for randomised controlled trials and non randomised
trials using the following keywords ‘fluid therapy OR crystalloids OR
colloids OR haemodynamic OR fluid responsiveness OR inotrops in
children OR cardiac output OR cardiac index OR oxygen delivery OR
oxygen delivery index OR oxygen consumption OR stroke volume
OR stroke volume variation OR pulse pressure variation OR mixed
venous oxygen saturation OR lactate OR oxygen extraction ratio OR
aortic velocity time integral variation OR aortic flow peak velocity
variation OR NIRS in children OR outcome in children OR mortality
in children OR morbidity in children OR length of hospital stay in
children OR randomised trials in children OR non randomised trials
in children’.
We used Medline (535009 identified titles), Embase (17536
identified trial titles), Central (37002 identified trial titles), Google
Scholar (1540 identified titles), Clinicaltrials.gov (504 identified
studies), Abstract Conference (0 titles identified) and DARE (566
identified titles) databases to search for titles, abstracts and complete
articles. Other sources like grey literature were also searched.
A flow chart illustrated the search and selection process as
recommended by the PRISMA (Preferred Reporting Items for
Systematic Reviews and Meta-analysis) statement [8,9] (Supplemental
Figure 1).
Statistic analysis:
-Comparisons (interventions) and outcomes (for mortality
and morbidity) were collected and analysed with Review Manager (RevMan) [Computer program]. Version 5.3.Copenhagen: The Nordic Cochrane Centre, the Cochrane
Collaboration, 2014.
Measures of the treatment effect (intervention effect) were
dichotomous for mortality and morbidity (number of deaths,
number of patients with complications) and was presented as OR
(odds ratio) with a 95% confidence interval (p<0.05 was considered
significant). Forest plots were used to provide visual summary of the
data included.
Forest plots and I2 statistics were used to assess for heterogeneity
in the studies. Funnel plots were used to assess for publication bias.
Sensitivity analysis was done by restricting the analysis to a
defined intervention and or to a subgroup of patients with a particular
outcome.
-A qualitative description and analysis for LOS was realized
since data concerning mean values were not always available in all
studies. Median values with Interquartile Ranges [IQR] for LOS were
compared between the experimental and the control groups using
Wilcoxon test (p<0.05 was considered significative) with XLSTAT
software 2018.3.
-The unit of analysis issues was the number of deaths for mortality
and the number of patients with complications for morbidity in the postoperative period until discharge from hospital and median
Length of Hospital Stay (LOS) in the postoperative period in days.
Missing data was not included:
The risk of bias in included studies was assessed using the
Oxford scale and the tools proposed by the Cochrane Handbook for
systematic reviews of interventions included with software [10].The level of evidence was assessed using the Grading of
Recommendation Assessment, Development, and Evaluation
(GRADE) system [11].
Results
33 complete trial articles were retained and analysed for inclusion.
10 studies were excluded because they did not meet the inclusion
criteria and 23 were included in the systematic review and metaanalysis.
See flowchart in (Supplemental Figure 1).
There were 23 studies included [12-34]. 3 were randomized
controlled trials, 12 were prospective observational and 8
retrospective observational trials. Studies found were most of them
observational and non interventional, see (Supplemental Tables 1-4)
for characteristics.
Effects of interventions:
I°) Regional oxygen saturation measured by NIRS: 8 studies
in cardiac surgery (3observational retrospective, 4 observational
prospective, 1 randomised controlled trial) were included for metaanalysis
[12,15-18,27-29].I.1°) Mortality (Supplemental Figure 2): 7 studies with 910
patients were included for this outcome. Mortality (OR=0.03 [0.01,
0.13], p<0.00001) was significantly lower in the experimental group.
The I2 statistics equaled 26 % and thus heterogeneity was very low. All
studies had bias (randomisation in the retrospective and prospective
trials, blinding and allocation concealment). Analysis with funnel
plot (Supplemental Figure 3) showed that the triangle was almost
symetrical; the risk of publication bias was present but low and can be
explained by the absence of studies which favored the control group
or studies without significant results.
Patients with perioperative higher (above baseline values)
regional oxygen saturations measured by NIRS in cardiac surgery had a favorable outcome in terms of mortality. The quality of evidence
(GRADE) was low (low heterogeneity and presence of bias in all
studies).
I.2°) Morbidity (organ dysfunction) (Supplemental Figure 4):
6 studies with 718 patients were included for this outcome. Organ
dysfunction (OR=0.02 [0.00, 0.37], p=0.009) was significantly lower
in the experimental group. Heterogeneity was high with I2 statistics
equaling 88%. Heterogeneity was due to the different number of
patients between the control and experimental groups. The risk of
bias in the studies was high (non randomisation, no blinding, No
allocation concealment). Analysis with funnel plot (Supplemental
Figure 5) showed a truncated triangle, publication bias was present
due to the absence of studies favoring the control group or with no
significant results. Patients with perioperative higher (above baseline
values) regional oxygen saturations measured by NIRS in cardiac
surgery had a favorable outcome in terms of organ dysfunction. The
quality of evidence GRADE) was low (high heterogeneity and bias in
all the studies).
I.3°) Morbidity (infections) (Supplemental Figure 6): one study
with 64 patients was included for this outcome (this outcome was not
evaluated in the other studies). There were less infections (Necrotizing
Enterocolitis) (OR= 0.03 [0.00, 0.60], p=0.02) in the experimental
group. Patients with perioperative higher (above baseline values)
regional splanchnic oxygen saturations measured by NIRS after
cardiac surgery had a favorable outcome in terms of infections
(Necrotizing Enterocolitis). The quality of evidence (GRADE) is very
low due to the small number of patients and the risk of bias.
There was no difference in LOS between the experimental and the
control groups when all the three studies were considered together.
The quality of evidence (GRADE) was very low (three studies
included and evaluated this outcome among the eight and high risk
of bias).
II°) Perioperative lactate levels: 9 studies in cardiac surgery
were included for meta-analysis (6 observational prospective and 3
observational retrospective) [19-24,30-32].
II.1°) Mortality (Supplemental Figure 7): 9 trials (6 prospective
and 3 retrospective) with 1690 children in cardiac surgery were included for this outcome. Mortality was significantly lower (OR=0.01
[0.00, 0.10], p=0.0003) in children with lower perioperative lactate
levels compared to those who had higher lactate levels. All studies had
bias (non randomisation, no blinding, no allocation concealment).
I2 statistics (91%) showed high heterogeneity among the studies due
to the different number of patients between the experimental and
the control groups. Funnel plot (Supplemental Figure 8) showed
a truncated triangle, thus publication bias was present due to the
absence of trials which favored control groups or with non significant
results. In cardiac surgical children with higher perioperative lactate
levels mortality was increased. The quality of evidence (GRADE) was
low (high heterogeneity and risk of bias).
II.2°) Morbidity (organ dysfunction) (Supplemental Figure
9) 6 studies (3 prospective and 3 retrospective) with 1241 children
were included for this outcome. Morbidity (OR= 0.01 [0.00, 0.16],
p=0.002) in terms of organ failure was lower in patients with lower
perioperative lactate levels. All studies had bias (non randomisation,
no blinding, no allocation concealment). I2 statistics (94%) showed
high heterogeneity due to the difference of number of patients
between the experimental and the control groups.Funnel plot
(Supplemental Figure 10) indicated that publication bias was present
due to the absence of trials with favorable outcome for control groups
or with non significant results. In cardiac surgical children with
higher perioperative lactate levels organ dysfunction was increased.
The quality of evidence (GRADE) was low (high heterogeneity and
risk of bias).
II.3°) Morbidity (infections) (Supplemental Figure 11): one study
with 255 children evaluated this outcome 31. Infections were lower
in the experimental group (OR= 0.00 [0.00, 0.06], p=0.0001). The
risk of bias was high (non randomised, no blinding, retrospective).
The quality of evidence (GRADE) is very low because only one study
among 9 was included for this outcome and the risk of bias was high.
II.4°) LOS (Supplemental Table 6): one study evaluated this
outcome [21], In this trial, LOS was lower in patients with lower
perioperative lactate levels. The quality of evidence (GRADE) was
very low (one study among 9 and high risk bias).
III°) Perioperative Lactate levels and ScVO2:
Three studies were included for meta-analysis (one prospective
and two retrospective).III.1°) Mortality (Supplemental Figure 12): three studies with 828
children in cardiac surgery evaluated this outcome using lactate levels
and ScVO2. In one study mortality was higher in the experimental
group and in the two other studies mortality was higher in the control
groups [23,31,32]. Taking the three studies together there was no
difference between the experimental and the control groups in terms
of mortality (OR=0.02 [0.00, 17.18], p=0.25).
All studies had bias (no randomisation, no blinding, no allocation
concealment). I2 statistics (95%) showed high heterogeneity due to
the difference between the number of patients in the experimental
and the control groups. Funnel plot (Supplemental Figure 13) showed
that publication bias was present due to the absence of trials favoring
experimental and control groups or studies with non significant
results. The quality of evidence (GRADE) was low due to the high risk of bias and heterogeneity.
III.2°) Morbidity (organ dysfunction) (Supplemental Figure
14): Two studies with 678 Children evaluated this outcome [31,32].
There was no difference in terms of organ dysfunction between
the experimental and the control group (OR= 0.01 [0.00, 4534.49],
p=0.5). All the two studies had bias (non randomisation, no
blinding, no allocation concealment). I2 statistics (98%) showed high
heterogeneity due to the difference of number of patients between the
experimental and the control groups.
Funnel plot (Supplemental Figure 15) showed that publication
bias was high due to the absence of trials favoring both experimental
and control groups or with non significant results. The quality
of evidence (GRADE) was low due to high risk of bias and high
heterogeneity.
III.3°) Morbidity (infections) (Supplemental Figure 16): one
study evaluated with 255 patients this outcome [31]. Infections were
lower in the experimental group (OR=0.00 [0.00, 0.06], p=0001). The
risk of bias was high (non randomised, no blinding, retrospective).
The quality of evidence (GRADE) was very low because only one
study among the three evaluated this outcome and bias was high.
III.4°) LOS was not evaluated.
IV°) ScVO2 (with Vigileo) + lactate levels: Two studies one
prospective and one retrospective with 405 children in cardiac
surgery were included [23,31].
IV.1°) Mortality (Supplemental Figure 17): Mortality was lower
in the experimental group (OR=0.00 [0.00, 0.02], p<0.0001). The
two studies had bias (non randomised, not blinded, no allocation
concealment) [23,31]. I2 (42%) statistics showed that heterogeneity
was low. Funnel plot (Supplemental Figure 18) showed that
publication bias was very high due to the absence of studies which
favored experimental and control groups or with non significant
results. The quality of evidence (GRADE) was low (high bias).
IV.2°) Morbidity (organ dysfunction) (Supplemental Figure 19)
one study evaluated this outcome [31]. Organ dysfunction was lower
in the experimental group (OR=0.00 [0.00, 0.00], p<0.00001). Quality
of evidence (GRADE) was low (only one study over two evaluated
this outcome).
IV.3°) Morbidity (infections) (Supplemental Figure 20): one
study evaluated this outcome [31].
Infections were lower in the experimental group (OR=0.00 [0.00,
0.06], p=0.0001). The quality of (GRADE) evidence was very low
(only one study over two evaluated this outcome and high bias).
IV.4°) LOS: not evaluated
V°) PiCCO system:
Two studies were identified and included a prospective trial in
cardiac surgery and a randomized controlled trial in severely burned
children [13,25].V.1°) Mortality (Supplemental Figure 21): one study in 152
children with severe burns evaluated this outcome [13]. There was
no difference in terms of mortality (OR= 0.56 [0.25, 1.26], p=0.16) between the experimental and the control groups. The risk of bias
was unclear: randomisation and the blinding were not clear. The
quality of evidence (GRADE) was very low because only one study
was included.
V.2°) Morbidity (organ dysfunction): this outcome was not
evaluated.
V.3°) Morbidity (infections) (Supplemental Figure 22): one
study evaluated this outcome in 152 severely burned children [13].
There was no difference in terms of infections (OR=0.49 [0.18, 1.31]
between the experimental and the contral groups. The risk of bias was
unclear: randomisation and blinding were not clear. The quality of
evidence (GRADE) is very low because only one study was included.
V.4°) LOS (Supplemental Table 7): the two studies evaluated this
outcome. LOS was not different between the two groups (p= 0.317).
The risk of bias was high (no randomisation, no blinding). The quality
of evidence (GRADE) is very low (only two studies included high risk
of bias).
VI°) Maximum vasoactive inotrop score (VIS):
VI.1°) Mortality (Supplemental Figure 23): two studies with 537
children in a prospective cardiac surgery trial and in a retrospective
study were included [26,33].Mortality (OR= 0.44 [0.18, 1.12], p=0.09) was not different
between the experimental and the control groups. The risk of bias was
high in the two studies (non randomisation, no blinding). The quality
of evidence (GRADE) was very low (only two studies found and high
risk of bias).
VI.2°) Morbidity (organ dysfunction) (Supplemental Figure 24):
The two studies were included. Morbidity (OR= 0.02 [0.00, 15.26],
p=0.25) in terms of organ failure was not different between the
experimental and the control groups. The risk of bias was high in the
two studies (non randomisation, no blinding). The I2 (96%) statistics
showed high heterogeneity due to the difference of the number of
patients between the experimental and the control groups. The
quality of evidence (GRADE) was very low.
VI.3°) Morbidity (infections) (Supplemental Figure 25), one
study was included [33]. Morbidity in terms of infections (OR=0.02
[0.00, 0.31], p=0.006) was lower in the experimental group. The risk
of bias was high (no randomisation, no blinding). The quality of
evidence (GRADE) was very low.
VI.4°) LOS (Supplemental Table 8) was evaluated in one study
and was low in the experimental group [33]. The risk of bias was high
(no randomisation, no blinding). The quality of evidence (GRADE)
is very low.
VII) Venous to arterial carbon dioxide difference:
One retrospective study with139 cardiac surgical patients
admitted to PICU was included [34].VII.1°) Mortality (Supplemental Figure 26): Mortality (OR=
0.01 [0.00, 0.12], p=0.0006) was lower in the experimental group. The
risk of bias was high (no randomisation, no blinding). The quality of
evidence (GRADE) was very low.
VII.2°) Morbidity (organ dysfunction) (Supplemental Figures
27- 29) organ failure (acute kidney failure p=0.005, ECMO p=0.004,
CPR p=0001) was significantly low in the experimental group. The
risk of bias was high (no randomisation, no blinding). The quality of
evidence (GRADE) very is low.
VII.3°) Morbidity (infections): was not evaluated.
VII.4°) LOS (Supplemental Table 9) was lower in the experimental
group. The quality of evidence was very low.
VIII) Transoesophageal doppler probe:
One randomised controlled trial with 14 children in scoliosis
surgery was found [14].VIII.1°) Mortality: was not evaluated.
VIII.2°) Morbidity (organ dysfunction) (Supplemental Figure
30): Organ dysfunction (OR=65.00 [2.24, 1887.35], p=0.02) (in terms
of acute kidney failure and alterations of motor evoked potentials)
was higher in the experimental group. The risk of bias was unclear
(in terms of randomisation, in terms of allocation concealment and
in terms of blinding), there was no compliance to the protocol. The
number of patients was too low. The quality of evidence (GRADE)
was very low.
VIII.3°) Mortality (infections): not evaluated.
VIII.4°) LOS: not evaluated.
IX.1°) all interventions included:
23 studies were identified with 3389 children. 3 studies were
randomised controlled. 12 prospective observational and 8 were
retrospective observational. 21 studies concerned 3223 cardiac
surgical children with congenital heart disease. Only two studies with
166 children concerned noncardiac patients: one study concerned
PiCCO in 152 children with severe burns and one concerned
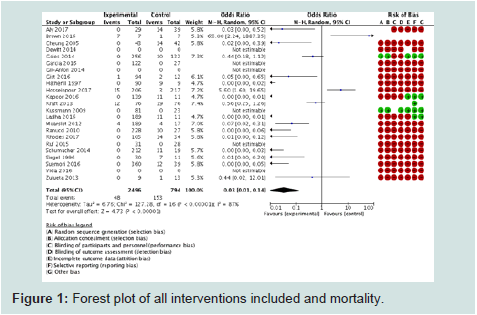
transoesophageal doppler in 14 children in scoliosis surgery.IX.1°) Mortality (Figure 1): 21 studies were included among the
23 identified with 3290 children. Mortality was significantly lower in
the experimental group (OR=0.03 [0.01, 0.14], p<0.00001). I2 statistics
(87%) showed high heterogeneity among the studies due to the
different number of patients in the experimental and control groups.
The risk of bias was high in all the studies (in terms of randomisation,
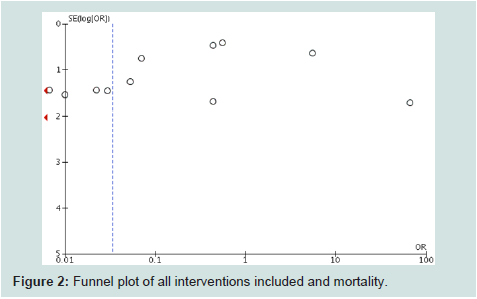
allocation concealment and blinding). Funnel plot (Figure 2) showed
an almost truncated triangle, suggesting that publication bias was
present due to the absence of trials which favored the control groups
or with non significant results. The quality of evidence (GRADE) was
low (high heterogeneity and bias).
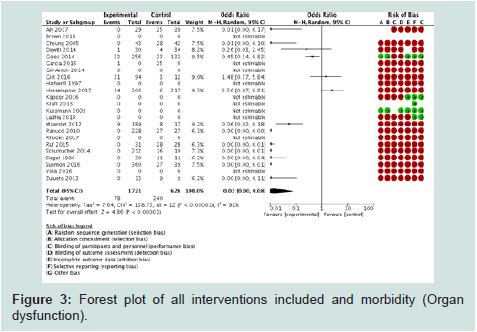
IX.2°) Morbidity (organ dysfunction) (Figure 3): 14 studies
among the 23 trials with 2347 children were included for this
outcome. Organ dysfunction was lower in the experimental group
(OR=0.02 [0.00, 0.08], p<0.00001). I2 statistics (91%) showed high
heterogeneity due to the difference in the number of patients between
the experimental and the control groups. The risk of bias was high
in all the studies (in terms of randomisation, allocation concealment
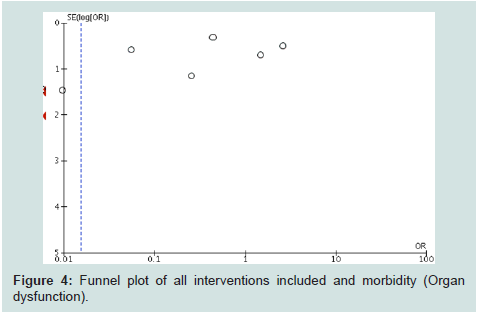
and blinding). Funnel plot (Figure 4) showed a truncated triangle
suggesting that publication bias was due to the absence of trials which
favored the control groups or with non significant results. The quality of evidence (GRADE) was low (high heterogeneity and bias).
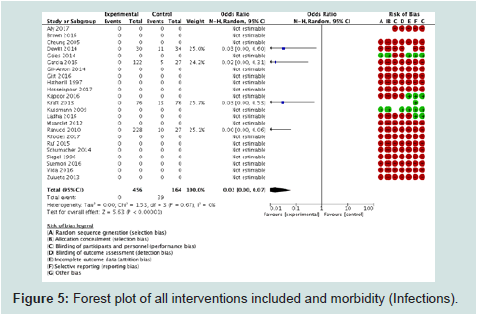
IX.3°) Morbidity (infections) (Figure 5): 4 studies among the
23 with 620 children were included for this outcome. Morbidity in
terms of infections was lower in the experimental group (OR= 0.02
[0.00, 0.07], p<0.00001. I2 statistics (0%) showed that there was no
heterogeneity among the studies. Bias was present in all studies in
terms of randomisation, allocation concealment and blinding. Funnel
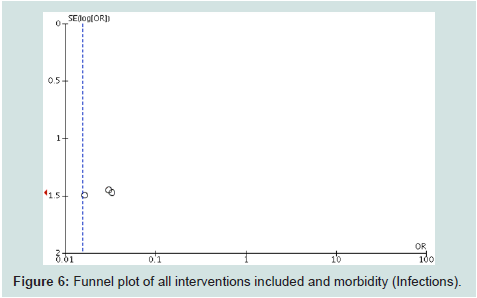
plot (Figure 6) showed a truncated triangle suggesting the presence
of publication bias due the absence of studies which favored control
or with non significant results. The quality of evidence was low (high
risk of bias) to moderate (no heterogeneity).
IX.4°) LOS (Supplemental Table 10): 8 studies evaluated this
outcome. LOS was significantly lower in the experimental group. Bias
was present in all studies (randomisation, allocation concealment
and blinding). The quality of evidence (GRADE) was very low (only 8
studies among 23 evaluated this outcome).
No trial was found concerning pulmonary artery catheter in
children and outcome.
One study concerning intraoperative transoesophageal
echocardiography was found but not included in the meta-analysis
because adults and children were included and thus did not meet
the inclusion criteria [35]. However this study found that LOS was
reduced in patients when a second intraoperative transoesophageal
echocardiography was performed.
Discussion
Overall completeness, applicability and quality of evidence:
This meta-analysis concerned children (under 18 years of age)
in the perioperative period (intraoperative up to the immediate
postoperative period i.e. first 24 hours postoperatively). The majority
of patients (more than 90%) were cardiac surgical patients (3223
children among 3389 patients). Only 166 children were non cardiac
patients (152 with severe burns and 14 in scoliosis surgery). Several
parameters were used to monitor haemodynamics intraoperatively
and or postoperatively in these patients : cerebral, renal, splanchnic,
somatic oxygen saturation; lactate levels and lactate clairance, mixed
central venous oxygen saturation via blood samples or using Vigileo,
cardiac output using PiCCO system and transoesophageal doppler,
venous to arterial carbon dioxide difference. Most of the studies were
retrospective and prospective observational (comparing outcome
between experimental groups where these parameters had optimal
values and control groups where these parameters were suboptimal).
Only three were interventional and applied stricto sensu goal directed
therapy protocols compared to standard care [13,14,32]. Kraft et al.
found no difference between the two groups;Hosseinpour et al. and Brown et al. found that outcome was
adverse in the experimental group but the quality of evidence was
very low due to the presence of bias in all the three studies and the
small number of patients.
Nevertheless, this systematic review and meta-analysis evidenced
that primary outcome (mortality and morbidity) was lower in cardiac
surgical children who had optimal haemodynamic parameter values
with overall low quality of evidence according to GRADE classification because of high risk bias and high heterogeneity. Secondary outcome
(Length of hospital stay, LOS) was also lower in the experimental
group but overall quality of evidence was very low because not all the
studies assessed this outcome and bias was present in all the studies.
In cardiac surgical children when haemodynamics were monitored
using the above mentioned variables or parameters (regional oxygen
saturation, mixed central venous oxygen saturation, lactate levels,
cardiac output, venous to arterial carbon dioxide difference) adverse
outcome was significantly higher in patients with suboptimal variable
values. Randomised controlled trials (RCT) where goal directed fluid
and haemodynamic therapy (GDFHT) using these parameters or
biomarkers should be developed to clarify the impact of this therapy
on postoperative outcome in cardiac pediatric patients since well
conducted prospective studies without bias concerning GDFHT in
this population are lacking. Trials using echocardiography to assess
postoperative outcome in cardiac or non cardiac pediatric patients are
lacking despite studies validating this device for fluid reponsiveness in
children under general anesthesia [36,37]. There was one retrospective
study which evaluated transoesophageal echocardiography during
congenital heart disease surgery and postoperative outcome but this
trial included adults and children and found LOS to be lower in the
group where there was a second intraoperative transoesophageal
echocardiograpy [35]. Concerning transoesophageal doppler, several
studies in non cardiac surgical children under general, caudal and
epidural anaesthesia have assessed cardiac output using this device
[38-40]. Studies concerning postoperative outcome with this device
in children are lacking ; the only study found was a trial in 14 scoliosis
surgery children where outcome was adverse in the transoesophageal
group but this study was stopped earlier because of non compliance
to the protocol and thus clinical evidence was very low for this trial
[14]; further studies should be conducted with the transoesophageal
doppler in children to clarify its impact on postoperative outcome ;
in adults it has been used in goal directed fluid and haemodynamic
therapy and has proven to reduce postoperative morbidity [41].
One study compared PiCCO and transoesophageal doppler in
PICU children and found that in non cardiac surgical patients the
two devices showed equivalent haemodynamic variables but this
observation was not evidenced in cardiac pediatric patients [42]. Two
studies evaluated PiCCO and outcome [13,25]; Kraft et al found that
mortality and morbidity was not different between the two groups ;
Gil-Anton et al found LOS to be lower in the experimental group ; the
quality of evidence was very low. More studies with less bias should
be developed with PiCCO in children intraoperatively to clarify its
impact on postoperative outcome.
Vigileo was used to monitor ScVO2 in two trials where mortality
was lower in patients with higher ScVO2 values, the quality of
evidence was low; organ failure and infections were lower in children
intraoperatively with higher ScVO2 values, the quality of evidence
was low [23,31]. More studies with less bias need to be developed with
vigileo in children to clarify its impact on postoperative outcome.
Limits:
Potential biases in the review process: Many studies had results
favoring the experimental groups. Studies which favored the control groups or with non significant results were lacking. Studies with
negative or positive results remain most of the time unpublished [43].
It is also important to publish these studies to reduce the publication
bias.Risk of bias in included studies: Allocation (selection bias) : was
not precised in most of the studies.
Blinding (performance bias and detection bias) : there was no
blinding in most of the studies.
Incomplete outcome data (attrition bias): almost all of the studies
except two studies did not precise the presence or the absence of
incomplete data [18,26].
Selective reporting (reporting bias): presenting exclusively studies
with favorable results could be a source of bias.
Other potential sources of bias: the different number of patients
in the experimental and control groups could be a source of bias.
Heterogeneity among the studies:
Strength of this study: 1) This trial clarified that intraoperative
goal directed fluid and hemodynamic therapy is not developed in
children. Future research will be conducted in developing trials with
less bias to assess its impact on outcome.2) Several parameters of adverse outcome in cardiac surgery have
been identified and should be integrated in goal directed fluid and
hemodynamic therapy research protocols with less bias to determine
their impact on outcome.
Conclusions and Recommendation
1°) Goal directed fluid and haemodynamic therapy in the
pediatric surgical population is not developed.
2°) In cardiac surgical patients intraoperative and postoperative
suboptimal values of regional oxygen saturation, mixed central
venous oxygen saturation, lactate levels, and venous to arterial carbon
dioxide difference are predictive of postoperative adverse outcome.
RCT where GDFHT using these parameters need to be developed
to clarify the impact of this practice on postoperative outcome in
children.
3°) In non cardiac surgical pediatric patients, research should be
directed in developing randomised controlled trials or prospective
trials with less biases to clarify the impact of intraoperative monitoring
with echocardiography, transoesophageal doppler, PiCCO, Vigileo to
guide fluid and haemodynamic therapy on postoperative outcome in
major surgery.
Acknowledgement
We would like to specify that the abstract of this article has been
accepted for presentation as a poster in the “Conference on
Principles of Pediatric Anesthesia and Critical Care, organized by
Havard Medical School in Boston on 10th May 2019