Journal of Nutrition & Health
Download PDF
Research Article
Evaluation of Bone Mineral Density and Biochemical Parameters of Bone Metabolism after Treatment with Biofield Energy Treated Vitamin D3 in MG-63 Cells
Singh J1, Trivedi MK1, Branton A1, Trivedi D1,Nayak G1, Mondal SC2 and Jana S2*
- 1Trivedi Global Inc, USA
- 2Trivedi Science Research Laboratory Pvt. Ltd, India
**Address for Correspondence: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Bhopal, India, E-mail: publication@trivedisrl.com
Citation: Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. Evaluation of Bone Mineral Density and Biochemical Parameters of Bone Metabolism after Treatment with Biofield Energy Treated Vitamin D3 in MG-63 Cells. J Nutri Health. 2018;4(1): 6.
Copyright © 2018 Singh J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Nutrition and Health | ISSN: 2469-4185 | Volume: 4, Issue: 1
Submission: 23 April 2018 | Accepted: 29 May 2018 | Published: 31 May 2018
Abstract
The aim of this study was to examine the potential of Consciousness Energy Healing based vitamin D3 and DMEM medium on bone health parameters. The test items (viz. vitamin D3 and DMEM), were divided into two parts. One part of each test item was received the Biofield Treatment by Jagdish Singh and those samples were labeled as Biofield Treated (BT) samples, while other parts of each sample were denoted as Untreated Test Items (UT). The test samples were found as safe in tested concentrations by MTT assay. ALP was significantly increased by 130.51%, 84.39%, and 67.51% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item, respectively at 1 μg/mL than UT-DMEM + UT-Test item group. Moreover, level of collagen was also significantly enhanced by 117.37% in BT-DMEM + UT-Test item at 100 μg/mL than untreated. Other parameter like collagen was significantly increased by 171.30%, 168.51%, and 110.48% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 50 μg/mL as compared to untreated. Additionally, level of collagen also elevated by 88.49%, 280.08%, and 63.28% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively, at 100 μg/mL compared to untreated. Besides, percent of bone mineralization was distinctly increased by 79.34%, 146.74%, and 162.76% in UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 100μg/mL compared to untreated. Overall, Biofield Treated vitamin D3 was significantly improved the bone health parameters and it could be a powerful alternative nutraceutical supplement to combat vitamin D3 deficiency and fight against various bone related problems including rickets, osteomalacia, osteoporosis, osteogenesis imperfect, bone fractures, osteoma, chondrodystrophia fetalis, stress management and prevention, autoimmune and inflammatory diseases, and antiaging by improving overall health.
Keywords
The Trivedi Effect; Biofield energy healing treatment; Osteosarcoma cells (MG-63); Collagen; Bone mineralization; Vitamin D3 deficiency
Abbreviations
MG-63: Human Bone Osteosarcoma Cells; ALP: Alkaline phosphatase; CAM: Complementary and alternative medicine; NHIS: National Health Interview Survey; NCCIH: National Center of Complementary and Integrative Health; DMEM: Dulbecco’s Modified Eagle’s Medium; FBS: Fetal Bovine Serum; ATCC: American Type Culture Collection; UT: Untreated; BT: Biofield Energy Treated; TI: Test Item
Acknowledgements
Authors are grateful to Dabur Research Foundation, Trivedi Global, Inc., Trivedi Science, Trivedi Testimonials and Trivedi Master Wellness for their support throughout the work.
Introduction
Vitamin D has multiple effects, which regulate the functions in different organs viz. brain, liver, lungs, heart, kidneys, skeletal, immune and reproductive systems. Moreover, it has significant anti-inflammatory, anti-aging, anti-stress, anti-arthritic, antiosteoporosis, anti-apoptotic, wound healing, anti-cancer, antipsychotic and anti-fibrotic actions [1]. Vitamin D receptors are widely distributed in most of the body organs viz. brain, liver, heart, lungs, kidney, pancreas, small and large intestines, muscles, reproductive, nervous system, etc. Vitamin D receptors influence cell-to-cell communication, normal cell growth, cell differentiation, cell cycling and proliferation, hormonal balance, neurotransmission process, skin health, immune and cardiovascular functions. In any living vertebrates, vitamin D plays an important role in maintaining a healthy skeletal structure and is essential for bone health. Naturally, it is synthesized in the presence of sunlight in the skin [2]. Most foods do not contain any vitamin D, additionally now-a-days due to aging, use of sunscreen, and change of zenith angle of sun the production of vitamin D3 has reduced [3]. Increasing age is not only related to a decrease in bone marrow depression and muscle strength but is also associated with marked changes in the immune and inflammatory responses [4]. Deficiency of vitamin D3 causes metabolic bone diseases like osteomalacia and exacerbates osteoporosis, etc. [5]. The quality of life for menopausal women is one of the most critical health problems in the today world. Metabolic bone disorders like osteoporosis are mainly prevalent in post-menopausal women. Hormonal factors and rapid bone loss in post-menopausal women leads to an increased risk of fractures [6]. Hence, the serum calcium and Alkaline Phosphatase (ALP) levels in post-menopausal women are the main two vital biochemical markers of bone metabolism. However, bone-specific ALP is the most important marker for osteoblast differentiation [7]. Further, it is generally accepted that an increased calcium intake along with an adequate source of vitamin D is important for maintaining good bone health. Vitamin D also plays an important role in maintaining an adequate level of serum calcium and phosphorus. Therefore, vitamin D has a great impact in forming and maintaining strong bones [8,9]. Bone strength depends on the quality, geometry, shape, micro architecture, turnover, mineral content, and the collagen content. Collagen is the major structural protein responsible for bone calcification. In the aging state, the mechanical properties of the bones become impaired and the bones get fragile, that causes various clinical disorders associated with bone collagen abnormalities and bone fragility, such as osteogenesis imperfect a and osteoporosis [10,11].
In recent years, several scientific reports and clinical trials have revealed the useful effects of Biofield Energy Treatments, which have shown to enhance immune function in cases of cervical cancer patients via therapeutic touch [12], massage therapy [13], etc. Complementary and Alternative Medicine (CAM) therapies are now rising as preferred models of treatment, among which Biofield Therapy (or Healing Modalities) is one approach that has been reported to have several benefits to enhance physical, mental and emotional human wellness. However, as per the data of 2012 from the National Health Interview Survey (NHIS), which indicated that the highest percentage (17.7%) of the Americans used dietary supplements as a complementary health approach as compared with other practices in past years. The National Center of Complementary and Integrative Health (NCCIH) has recognized and accepted Biofield Energy Healing as a CAM health care approach in addition to other therapies, medicines and practices such as natural products, deep breathing, yoga, Tai Chi, Qi Gong, chiropractic/osteopathic manipulation, meditation, massage, special diets, homeopathy, progressive relaxation, guided imagery, acupressure, acupuncture, relaxation techniques, hypnotherapy, healing touch, movement therapy, pilates, Rolfing structural integration, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines, naturopathy, essential oils, aromatherapy, Reiki, and cranial sacral therapy. Human Biofield Energy has subtle energy that has the capacity to work in an effective manner [14]. CAM therapies have been practiced worldwide with reported clinical benefits in different health disease profiles [15]. This energy can be harnessed and transmitted by the experts into living and non-living things via the process of Biofield Energy Healing. Biofield Energy Treatment (The Trivedi Effect®) has been published in numerous peer-reviewed science journals with significant outcomes in many scientific fields such as cancer research [16,17], microbiology [18-21], biotechnology [22,23], pharmaceutical science [24-27], agricultural science [28-31], materials science [32-35], nutraceuticals [36,37], skin health, human health and wellness.
Materials and Methods
Chemicals and reagents
Antibiotic solution (penicillin-streptomycin) was procured from HiMedia, India, while 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2Htetrazolium) (MTT), Direct Red 80, and Ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma, USA. Fetal Bovine Serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Life Technology, USA. Rutin hydrate was purchased from TCI, Japan, while vitamin D3 (denoted as test item) and L-ascorbic acid were obtained from Sigma-Aldrich, USA. All the other chemicals used in this experiment were analytical grade procured from India
Cell culture
The human bone osteosarcoma cells (MG-63) were used as the test system in the current study. The MG-63 cells were maintained under the DMEM growth medium for routine culture and supplemented with 10% FBS. Growth conditions were maintained as 37 °C, 5% CO2 and 95% humidity and sub-cultured by trypsinisation followed by splitting the cell suspension into fresh flasks and supplementing with fresh cell growth medium. Three days before the start of the study (i.e., day -3), the growth medium of near-confluent cells was replaced with fresh phenol-free DMEM, supplemented with 10% charcoal dextran stripped FBS (CD-FBS) and 1% penicillin-streptomycin [38].
Experimental design
The experimental groups consisted of cells in baseline control (untreated cells), vehicle control groups (0.05% DMSO with Biofield Energy Treated and untreated DMEM), a positive control group (rutin hydrate) and experimental test groups. The experimental groups included the combination of the Biofield Energy Treated and untreated vitamin D3/DMEM. It consisted of four major treatment groups on specified cells with UT-DMEM + UT-Test item, UTDMEM + Biofield Energy Treated test item (BT-Test item), BTDMEM + UT-Test item, and BT-DMEM + BT-Test item.
Consciousness energy healing treatment strategies
The test item (vitamin D3) and DMEM were divided into two parts. One part each of the test item and DMEM were treated with the Biofield Energy (also known as The Trivedi Effect®) and coded as the Biofield Energy Treated items, while the second part did not receiveany sort of treatment and was defined as the untreated samples. This Biofield Energy Healing Treatment was provided by Jagdish Singh, who participated in this study and performed the Biofield Energy Healing Treatment remotely for ~5 minutes. Biofield Energy Healer was remotely located in the USA, while the test samples were located in the research laboratory of Dabur Research Foundation, New Delhi, India. The Biofield Energy Treatment was administered for 5 minutes through the healer’s unique Energy Transmission process remotely to the test samples under laboratory conditions. Biofield Energy Healer (Jagdish Singh) in this study never visited the laboratory in person, nor had any contact with the Test item and medium. Further, the control group was treated with a sham healer for comparative purposes. The sham healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions for experimental study.
Determination of non-cytotoxic concentration
The cell viability test was performed by MTT assay in the human bone osteosarcoma cell line (MG-63). The cells were counted and plated in 96-well plates at the density corresponding to 5 X 103 to 10 X 103 cells/well/180 μL of cell growth medium. The above cells were incubated overnight under growth conditions and allowed cell recovery and exponential growth, and then they were subjected to serum stripping or starvation. The cells were treated with the testitem, DMEM, and the positive control. The untreated cells served as baseline control. The cells in the above plate (s) were incubated for a time point ranging from 24 to 72 hours in CO2 incubator at 37 °C, 5% CO2 and 95% humidity. Following incubation, the plates were taken out and 20 μL of 5 mg/mL of MTT solution was added to all the wells followed by an additional incubation for 3 hours at 37 °C. The supernatant was aspirated and 150 μL of DMSO and was added to each well to dissolve formazan crystals. The absorbance of each well was read at 540 nm using a Synergy HT micro plate reader, BioTek, USA. The percentage cytotoxicity at each tested concentration of the test substance was calculated using the following Equation 1:
% Cytotoxicity = {(1-X)/R}*100----------------------- (1)
Where, X = Absorbance of treated cells; R = Absorbance of untreated cells
The percentage cell viability corresponding to each treatment was then being obtained using the following Equation 2:
% Cell Viability = 100 - % Cytotoxicity----------------------- (2)
The concentrations exhibiting ≥70% Cell viability was considered as non-cytotoxic [39].
Assessment of Alkaline Phosphatase (ALP) activity
The cells were counted using a hemocytometer and plated in a 24-well plate at the density corresponding 1 x 104 cells/well in phenol free DMEM supplemented with 10% CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37 °C, 5% CO2 and 95% humidity. After 48 hours of incubation, the plate was taken out and processed for the measurement of ALP enzyme activity. The cells were washed with 1X PBS and lysed by freeze thaw method i.e., incubation at -80 °C for 20 minutes followed by incubation at 37 °C for 10 minutes . To the lysed cells, 50 μL of substrate solution i.e., 5 mM of p-Nitrophenyl Phosphate (pNPP) in 1M diethanolamine and 0.24 mM magnesium chloride (MgCl2) solution (pH 10.4) was added to all the wells followed by incubation for 1 hour at 37 °C. The absorbance of the above solution was read at 405 nm using Synergy HT micro plate reader (Biotek, USA). The absorbance values obtained were normalized with substrate blank (pNPP solution alone) absorbance values. The percentage increase in ALP enzyme activity with respect to the untreated cells (baseline group) was calculated using Equation 3:
% Increase in ALP = {(X-R)/R}*100----------------------- (3)
Where,
X = Absorbance of cells corresponding to positive control and test groups
R = Absorbance of cells corresponding to baseline group (untreated cells)
Assessment of collagen synthesis
The MG-63 cells were counted using a hemocytometer and plated in 24-well plate at the density corresponding to 10 x 103 cells/well in phenol-free DMEM supplemented with 10% CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37 °C, 5% CO2 and 95% humidity. After 48 hours of incubation, the plate was taken out and the amount of collagen accumulated in MG-63 cells corresponding to each treatment was measured by Direct Sirius red dye binding assay. In brief, the cell layers were washed with PBS and fixed in Bouin’s solution (5% acetic acid, 9% formaldehyde and 0.9% picric acid) for 1 hour at Room Temperature (RT). After 1 hour of incubation, the above wells were washed with mille Q water and air dried. The cells were then stained with Sirius red dye solution for 1 hour at RT followed by washing in 0.01 N HCl to remove unbound dye. The collagen dye complex obtained in the above step was dissolved in 0.1 N NaOH and absorbance was read at 540 nm using Biotek Synergy HT micro plate reader. The level of collagen was extrapolated using standard curve obtained from purified Calf Collagen Bornstein and Traub Type I (Sigma Type III). The percentage increase in collagen level with respect to the untreated cells (baseline group) was calculated using Equation 4:
% Increase in collagen levels = {(X-R)/R}*100-------------------(4)
Where,
X = Collagen levels in cells corresponding to positive control and test groups
R = Collagen levels in cells corresponding to baseline group (untreated cells)
Assessment of bone mineralization by alizarin red s Staining
The MG-63 cells were counted using a hemocytometer and plated in 24-well plate at the density corresponding to 10 x103 cells/well in phenol free DMEM supplemented with 10% CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37 °C, 5% CO2 and 95% humidity to allow cell recovery and exponential growth. Following overnight incubation, the above cells were subjected to serum stripping for 24 hours. The cells were then treated with non-cytotoxic concentrations of the test samples and positive control. After 3 to 7 days of incubation with the test samples and positive control, the plates were taken out, cell layers processed further by staining with Alizarin Red S dye. The cells were fixed in 70% ethanol for 1 hour, after which Alizarin Red solution (40 μm; pH 4.2) was added to the samples for 20 minutes with shaking. The cells were washed with distilled water to remove unbound dye. For quantitative analysis by absorbance evaluation, nodules were solubilized with 10% cetylpyridinium chloride for 15 minutes with shaking. Absorbance was measured at 562 nm using Biotek Synergy HT micro plate reader. The percentage increase in bone mineralization with respect to the untreated cells (baseline group) was calculated using the following Equation 5:
% Increase = {(X-R)/R}*100----------------------- (5)
Where,
X = Absorbance in cells corresponding to positive control or test
groups; R = Absorbance in cells corresponding to baseline (untreated) group.
Statistical analysis
All the values were represented as percentage of the respective parameters. For statistical analysis Sigma-Plot (version 11.0) was used as a statistical tool. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
MTT assay
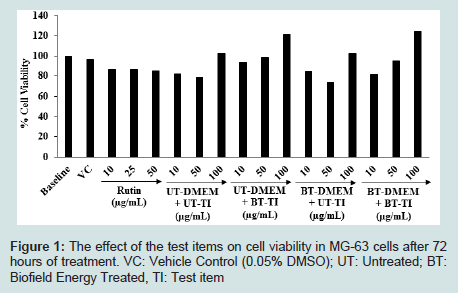
Cell-based assays are used for the assessment of cell proliferation or cytotoxicity [40,41]. The MTT tetrazolium assay has been widely used for the screening of viable cells [42]. Hence, in this experiment authors used MTT cell viability assay as a systemic tool for the assessment of viable cells count of the test samples in MG-63 cell line. The results of cell viability after treatment with the test samples in MG-63 cells are shown in (Figure 1). The data were expressed as percentage, did not show any cytotoxicity (as evidence of cell viability approximately greater than 73%) across all the tested concentrations as maximum of 100 μg/mL. Therefore, the safe concentrations were used in this experiment to study the effect of the test samples on the levels of Alkaline Phosphatase (ALP) activity, collagen synthesis, and bone mineralization in MG-63 cells.
Figure 1: The effect of the test items on cell viability in MG-63 cells after 72 hours of treatment. VC: Vehicle Control (0.05% DMSO); UT: Untreated; BT:Biofield Energy Treated, TI: Test item
MTT assay
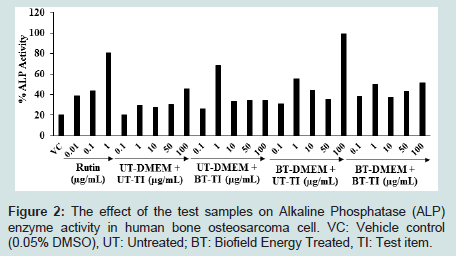
The effect of the test substances on ALP activity in MG-63 cells is shown in Figure 2. The level of ALP was increased by 20.50% in the Vehicle Control (VC) group compared to the untreated cells group. The ALP activity was increased by 38.78%, 43.61%, and 80.92% in the positive control group at the concentration of 0.01, 0.1, and 1 μg/mL, respectively in a dose-dependent manner compared to the untreated cells group. The level of ALP was significantly increased by 26.44%, 52.83%, and 84.93% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item group, at the concentration of 0.1 μg/mL compared to the UT-DMEM + UT-Test item group. ALP level was further significantly increased by 130.51%, 84.39%, and 67.51% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item group, at the concentration of 1 μg/mL compared to the UT-DMEM + UT-Test item group. Moreover, at 10 μg/mL the level of ALP was significantly enhanced by 19.29%, 59.29%, and 37.08% in the UT- DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively compared to the UT-DMEM + UT-Test item group. Additionally, the level of ALP was significantly increased by 13.37%, 17.81%, and 42.01% in the UT-DMEM + BTTest item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 50 μg/mL compared to the UT-DMEM + UT-Test item group. At higher concentration (100 μg/mL), the level of ALP was also significantly increased by117.37% and 12.29% in the BT-DMEM + UT-Test item and BT-DMEM + BT-Test item groups, respectively than untreated group (Figure 2). Overall, the Consciousness Energy Healing Treated (The Trivedi Effect®) test item group (i.e., vitamin D3) showed an improved synthesis of ALP level in the human osteosarcoma cells with respect to the untreated item items group at 50 μg/mL. The ALP activity is essential for the bone mineralization and considered a useful biochemical marker for bone formation [43]. Here, it was revealed that the Consciousness Energy Healing Treated vitamin D3 significantly increased the level of ALP expression, which might be very helpful to the patients suffering from various bone-related disorders.
Figure 2: The effect of the test samples on Alkaline Phosphatase (ALP) enzyme activity in human bone osteosarcoma cell. VC: Vehicle control (0.05% DMSO), UT: Untreated; BT: Biofield Energy Treated, TI: Test item.
Assessment of collagen activity
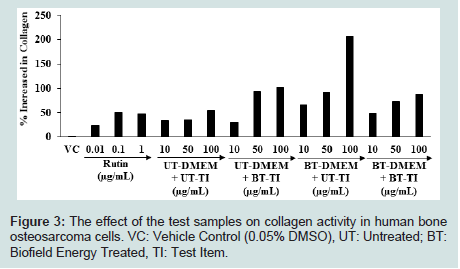
The effect of the test samples on the collagen activity in MG-63 cell line is shown in Figure 3. The Vehicle Control group (VC) showed the level of collagen activity as 2.2% compared to the untreated cells group. The synthesis of collagen was significantly increased by 24%, 50.29%, and 47.71% at 0.01, 0.1, and 1 μg/mL, respectively in the positive control (rutin) group compared to the untreated cells group. The collagen synthesis was significantly increased by 95.03% and 43.97% in the BT-DMEM + UT-Test item and BT-DMEM + BT-Test item groups, respectively at 10 μg/mL, compared to the UT-DMEM + UT-Test item group. Moreover, the level of collagen was significantly increased by 171.30%, 168.51%, and 110.48% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BTDMEM + BT-Test item groups, respectively at 50 μg/mL, compared to the UT-DMEM + UT-Test item group. Additionally, at 100 μg/mL collagen concentration was significantly elevated by 88.49%, 280.08%, and 63.28% in the in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively, compared to the UT-DMEM + UT-Test item group (Figure 3).
Figure 3: The effect of the test samples on collagen activity in human bone osteosarcoma cells. VC: Vehicle Control (0.05% DMSO), UT: Untreated; BT: Biofield Energy Treated, TI: Test Item.
Altogether, the Consciousness Energy Healing based test item group (i.e., vitamin D3) showed an improved synthesis of collagen content in the human osteosarcoma cells with respect to all the treatment groups. Type I collagen is the major structural protein in extra cellular matrix component responsible for bone calcification and also promoting osteoblast differentiation [44]. Here, the Biofield Energy Treated vitamin D3 significantly improved the level of collagen which could be beneficial to maintain a health bone. Overall, The Trivedi Effect® - Consciousness Energy Healing Treatment modality showed a significant improvement of the collagen level in human osteosarcoma cells. Thus, it is assumed that The Trivedi effect® has the potential to improve the bone health in various skeletal disorders.
Bone mineralization
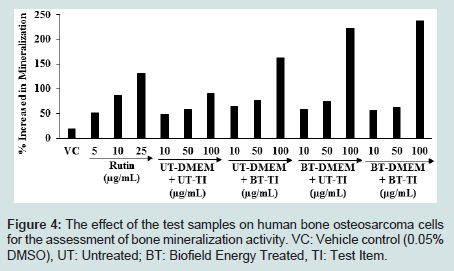
Reduction of bone properties and an increased the risk of bonefracture is due to deficiency of vitamin-D and calcium [45]. Moreover, vitamin D co-regulates calcium homeostasis by influencing intestinal calcium absorption, renal calcium reabsorption and bone resorption by osteoclasts [46,47]. The effect of test items on bone mineralization in MG-63 cells is shown in Figure 4. The vehicle control group showed 19.4% increased the bone mineralization as compared to the untreated cells group. The positive control (rutin) showed 50.46%, 86.16%, and 130.60% increased of percent bone mineralization at 5,10 and 25 μg/mL, respectively compared to the untreated cells group in a concentrationdependent manner. The percent bone mineralization was significantly raised by 31.62%, 19.89%, and 16.32% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item group, respectively at 10 μg/mL compared to the UT-DMEM + UTTest item group. Further, a noticeably increased the percentage of bone mineralization was observed by 30.07%, 27.95%, and 6.78% in the UTDMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 50 μg/mL with respect to the UT-DMEM + UT-Test item group. Further, at 100 μg/mL the percent of bone mineralization was remarkably increased by 79.34%, 146.74%, and 162.76% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively with respect to the UT-DMEM + UT-Test item group (Figure 4). Thus, based on the above findings it is hypothesized that the Consciousness Energy Healing Treatment (The Trivedi Effect®) based test item groups (i.e., vitamin D3) showed a remarkable improvement of bone mineralization content assessed by in vitro in the human osteosarcoma cells (MG-63) with respect to the all others treatment groups. Figure 4: The effect of the test samples on human bone osteosarcoma cells for the assessment of bone mineralization activity. VC: Vehicle control (0.05% DMSO), UT: Untreated; BT: Biofield Energy Treated, TI: Test Item.
Conclusion
The MTT cell viability assay data showed more than 73% cells were viable, which indicated that the test samples were safe and nontoxic in all the tested concentrations. ALP was significantly increased by 130.51%, 84.39%, and 67.51% in the UT-DMEM + BTTest item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item, respectively at 1 μg/mL than the UT-DMEM + UT-Test item group. Further, the level of collagen was also significantly enhanced by 117.37% in the BT-DMEM + UT-Test item at 100 μg/mL than the untreated group. Collagen was significantly increased by 171.30%, 168.51%, and 110.48% in the UT-DMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively at 50 μg/mL compared to the untreated group. Additionally, the level of collagen was also elevated by 88.49%, 280.08%, and 63.28% in the UTDMEM + BT-Test item, BT-DMEM + UT-Test item, and BT-DMEM + BT-Test item groups, respectively, at 100 μg/mL compared to the untreated group. Altogether, the Biofield Energy Treated test samples (The Trivedi Effect®) demonstrated a significant impact on bone health parameters. Therefore, the Consciousness Energy Healing based vitamin D3 might be suitable for the development of an alternative and more effective supplement for vitamin D3 deficiency, which could be useful for the management of various bone related disorders viz. low bone density and osteoporosis, osteogenesis imperfect a, Paget’s disease of bone, rickets, osteomalacia, bone and joint pain, bone fractures, deformed bones, osteoma, chondrodystrophia fetal is, etc. Besides, it can also be utilized in organ transplants (for example kidney transplants, liver transplants and heart transplants), various autoimmune disorders such as Lupus, Addison Disease, Celiac Disease (gluten-sensitive enteropathy), Dermatomyositis, Graves’ Disease, Hashimoto Thyroiditis, Multiple Sclerosis, Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Reactive Arthritis, Rheumatoid Arthritis, Sjogren Syndrome, Systemic Lupus Erythematosus, Type 1 Diabetes, Alopecia Areata, Crohn’s Disease, Fibromyalgia, Vitiligo, Psoriasis, Scleroderma, Chronic Fatigue Syndrome and Vasculitis. Further it also is useful in various inflammatory disorders such as Asthma, Ulcerative Colitis, Alzheimer’s disease, Atherosclerosis, Dermatitis, Diverticulitis, Hepatitis, and Irritable Bowel Syndrome. Additionally, Trivedi’s propritory Therapy would be very effective as an anti-stress, anti-arthritic, anti-osteoporosis, anti-apoptotic, wound healing, anti-cancer, anti-psychotic and anti-fibrotic actions stress management and prevention, and anti-aging by improving overall health, Parkinson’s Disease and stress etc. to modulate the immune system by improving overall health.
References
- Holick MF (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases cancers, and cardiovascular disease. Am J Clin Nut 80: 1678S-1688S.
- Holick MF (1996) Vitamin D and bone health. J Nutr 126: 1159S-1164S.
- Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF (1987) Sunscreens suppress vitamin D3 synthesis. J Clin Endocrinol Metab 64: 1165-1168.
- 4.Barnes MS, Robson JP, Bonham MP, Strain J, Wallace J (2006) Vitamin D: Status, supplementation and immunomodulation. Cur Nut Food Sci 2: 315-336.
- Laird E, Ward M, McSorley E, Strain JJ, Wallace J (2010) Vitamin D and bone health; Potential mechanisms. Nutrients 2: 693-724.
- Bhattarai T, Bhattacharya K, Chaudhuri P, Sengupta P (2014) Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women. Malays J Med Sci 21: 58-61.
- Iba K, Takada J, Yamashita T (2004) The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab 22: 594-596.
- Holick MF, Garabedian M (2006) Vitamin D: Photobiology, metabolism, mechanism of action, and clinical applications. Primer on the metabolic bone diseases and disorders of mineral metabolism. Edited by: Favus MJ, Washington, DC pp: 129-137.
- DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80: S1689-S1696.
- Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17: 319-336.
- Sroga GE, Vashishth D (2012) Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10: 141-150.
- Lutgendorf SK, Mullen-Houser E, Russell D, Degeest K, Jacobson G, et al. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun 24: 1231-1240.
- Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, et al. (1996) Massage therapy is associated with enhancement of the immune system's cytotoxic capacity. Int J Neurosci 84: 205-217.
- Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R, et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med 4: 58-66.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8: 703-717.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antibiogram, biochemical reactions and biotyping of biofield treated Providencia rettgeri. Am J Health Res 3: 344-351.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antimicrobial sensitivity, biochemical characteristics and biotyping of Staphylococcus saprophyticus: An impact of biofield energy treatment. J Women’s Health Care 4: 271.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Shettigar H, et al. (2015) Antimicrobial susceptibility pattern, biochemical characteristics and biotyping of Salmonella paratyphi A: An impact of biofield treatment. Clin Microbiol 4: 215.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antibiogram of biofield-treated Shigella boydii: Global burden of infections. Scie J Clin Med 4: 121-126.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of antibiogram, genotype and phylogenetic analysis of biofield treated Nocardia otitidis. Biol Syst Open Access 4: 143.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield. Pharm Anal Acta 6: 395.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med Chem 5: 340-344.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Effect of biofield treatment on spectral properties of paracetamol and piroxicam. Chem Sci J 6: 98.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Effect of biofield treatment on spectral properties of paracetamol and piroxicam. Chem Sci J 6: 98.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). J Food Nutr Sci 3: 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Agronomic characteristics, growth analysis, and yield response of biofield treated mustard, cowpea, horse gram, and groundnuts. Int J Genetics and Genomics 3: 74-80.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Analysis of genetic diversity using simple sequence repeat (SSR) markers and growth regulator response in biofield treated cotton (Gossypium hirsutum L.). Am J Agricul Fores 3: 216-221.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Evaluation of vegetative growth parameters in biofield treated bottle gourd (Lagenaria siceraria) and okra (Abelmoschus esculentus). Int J Nutr Food Sci 4: 688-694.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Evaluation of atomic, physical, and thermal properties of bismuth oxide powder: An impact of biofield energy treatment. Am J Nano Res Appl 3: 94-98.
- Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015) Influence of biofield treatment on physical, structural and spectral properties of boron nitride. J Material Sci Eng 4: 181.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Characterization of physical and structural properties of brass powder after biofield treatment. J Powder Metall Min 4: 134.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Evaluation of biofield treatment on physical and structural properties of bronze powder. Adv Automob Eng 4: 119.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Jana S, et al. (2015) Bio-field treatment: An effective strategy to improve the quality of beef extract and meat infusion powder. J Nutr Food Sci 5: 389.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Biofield treatment: A potential strategy for modification of physical and thermal properties of gluten hydrolysate and ipomoea macroelements. J Nutr Food Sci 5: 414.
- Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for in vitro research. Eur Cells Mater 24: 1-17.
- Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity (ISO 10993-5:2009), I.S.EN ISO, 10993-5: 20093.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth 65: 55-63.
- Marshall NJ, Goodwin CJ, Holt SJ (1995) A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul 5: 69-84.
- Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, et al. (2016) Cell Viability Assays. In: Sittampalam GS, Coussens NP, Brimacombe K, et al., editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004.
- Gerald J. Atkins, David M. Findlay, Paul H (2011) Anderson, Howard A. Morris. Vitamin D (3rd Eds), Vitamin D 1: 411-424.
- Oishi Y, Fu ZW, Ohnuki Y, Kato H, Noguchi T (2002) Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: Effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br J Dermatol 147: 859-868.
- Lips P, van Schoor NM (2011) The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab 25: 585-591.
- Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, et al. (2010) Bone mineralization defects and vitamin D defciency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 25: 305-312.
- Hossein-Nezhad A, Holick MF (2013) Vitamin D for health: A global perspective. Mayo Clin Proc 88: 720-755.