Journal of Neurology and Psychology
Download PDF
Research Article
*Address for Correspondence: Abdel-Salam OA, Department of Neurology, Faculty of Medicine, Mansoura University, Mansoura, Daqahlia 35516, Egypt, E-mail: osama.neuro2003@gmail.com
Citation: Alhalby A, Abdel-Salam OA, Ahmed ZM. Weight Reduction Aids Antiepileptic Therapy to Restore Ovarian Functions of Epileptic Polycystic Ovary Women. J Neurol Psychol. 2014;2(1): 6.
Copyright © 2013 Alhalby A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 2, Issue: 1
Submission: 20 January 2014 | Accepted: 15 February 2014 | Published: 20 February 2014
Reviewed & Approved by: Dr. Maromi Nei, Department of Neurology, Thomas Jefferson University Hospital, USA
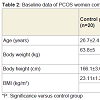
Studied patients showed non-significant difference as regards body weight, body height and body mass index; while all showed significantly higher body weight and body mass index compared to control group (Table 2).
Weight reduction regimen for 6-months resulted in significant reduction of body weight and BMI of PCOS women compared to their measures at the start of the regimen with non-significant difference among studied groups as regards both body weight and BMI determined at the end of the weight reduction regimen (Table 3).
Weight Reduction Aids Antiepileptic Therapy to Restore Ovarian Functions of Epileptic Polycystic Ovary Women
Alhalby A1, Abdel-Salam OA2* and Ahmed ZM3
- 1Department of Gynecology and Obstetrics, Faculty of Medicine, Menoufya University, Egypt
- 2Department of Neurology, Faculty of Medicine, Mansoura University, Egypt
- 3Department of Neurology, Faculty of Medicine, Al-Azhar University, Egypt
*Address for Correspondence: Abdel-Salam OA, Department of Neurology, Faculty of Medicine, Mansoura University, Mansoura, Daqahlia 35516, Egypt, E-mail: osama.neuro2003@gmail.com
Citation: Alhalby A, Abdel-Salam OA, Ahmed ZM. Weight Reduction Aids Antiepileptic Therapy to Restore Ovarian Functions of Epileptic Polycystic Ovary Women. J Neurol Psychol. 2014;2(1): 6.
Copyright © 2013 Alhalby A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology and Psychology | ISSN: 2332-3469 | Volume: 2, Issue: 1
Submission: 20 January 2014 | Accepted: 15 February 2014 | Published: 20 February 2014
Reviewed & Approved by: Dr. Maromi Nei, Department of Neurology, Thomas Jefferson University Hospital, USA
Abstract
Objectives: To evaluate the hormonal profile of epileptic women with polycystic ovary (PCOS) and to determine the impact of weight reduction regimen on their ovulatory function in comparison to nonepileptic PCOS women and normal fertile control women.Patients and Methods: The study included 60 PCOS diagnosed depending on the Rotterdam criteria, 40 women were epileptics and 20 women were non-epileptics. The epileptic disorders were classified in accordance with the International League Against Epilepsy (ILAE) Classification and treatment 2010, remained constant throughout the whole study period. In all patients transvaginal ultrasonographic (TVU) examination was performed and body weight (BW) and height were measured to calculate body mass index (BMI). Blood samples were obtained in the early follicular phase of the baseline cycle (days 5-7) for hormonal profile determination. All PCOS women were prescribed the same energy-restricted, high-protein diet and underwent a walking/jogging program comprising five sessions per week for 6 months and hormonal profile was repeated.
Results: Among women with epilepsy, 25 patients had cryptogenic generalized epilepsy and 15 patients had cryptogenic localization-related epilepsy, 24 patients were on therapy and 16 were untreated. All studied patients showed significantly higher BW and BMI compared to control group. Weight reduction regimen resulted in significant reduction of BW and BMI of PCOS women compared to baseline measures with non-significant difference among studied groups. Baseline estimation of serum hormones profile showed discrepant results that were significantly deviated in all studied women from the control levels. The impact of epileptic therapy was evident and manifested as significantly lower FSH, LH and prolactin (PRL) in untreated women compared to controls, non-epileptic patients and epileptic patients receiving treatment. Serum E2 showed significant increase in non-epileptic patients, but was non-significantly increased in epileptic patients compared to baseline levels. Patients maintained on VPA showed significantly higher serum FSH, PRL and testosterone with significantly lower serum E2 compared to those receiving other forms of antiepileptic therapy. Nineteen women got ovulation with success rate of 31.7%; 11 women were non-epileptic, 6 women on antiepileptic therapy and only 2 of women who were untreated.
Conclusion: In epileptic PCOS women when polytherapy was provided with avoidance of VPA, combined weight reduction regimens associated with aerobic physical exercise intervention induce weight loss with subsequent amelioration of inhibitory effect of obesity on the reproductive function and adjustment of the hyperandrogenic milieu and induction of ovulatory cycles that promise for regaining fertility.
Introduction
Epilepsy is a major public health problem in all countries. It has a strong personal, familial, and social impact, and in addition to seizures, patients and their families may have to face many other problems. For instance, impaired fertility is an additional problem of women with epilepsy with increased frequency of reproductive endocrine disorders, in particular of polycystic ovary syndrome [1].Polycystic ovary syndrome is associated with a number of reproductive disorders and is characterized by the presence of polycystic ovaries, menstrual dysfunction, infertility or reduced fertility, and biochemical or clinical hyperandrogenism. PCOS also increases the prevalence and risk of a number of cardiometabolic disturbances including insulin resistance, hypertension, dyslipidemia, and diabetes [2].
The relationships among hormones, epilepsy, and the medications used to treat epilepsy are complex, with tridirectional interactions that affect both men and women in various ways. Abnormalities of baseline endocrine status occur more commonly in people with epilepsy. Abnormalities are most often described for the sex steroid hormone axis, commonly presenting as sexual dysfunction in men and women with epilepsy and lower fertility [3,4].
Verrotti et al. reported that epileptic discharges from the temporal lobe may have a direct influence on the function of the hypothalamic-pituitary axis, thus altering the release of sex steroid hormones, including the production of LH, FSH, gonadotropinreleasing hormone and prolactin [5].
Also, Luef indicated that temporal lobe epilepsy is associated with abnormalities of reproductive physiology, but the mechanisms of hormonal dysregulation are not clear [6]. A direct influence of epilepsy on the reproductive endocrine system is suggested by acute changes in PRL and gonadotropin levels following generalized and partial seizures, pointing to a possible relationship between temporolimbic epileptiform discharges and particular reproductive endocrine disorders. Chronic effects of the epileptic state and the acute impact of seizures could alter hypothalamic function, as indicated by downstream pulsatile secretion of luteinizing hormone. Brain controls reproductive function primarily through hypothalamic regulation of pituitary secretion regions of the hypothalamus. These are areas receive extensive direct connections from the cerebral hemispheres, especially from temporolimbic structures, most notably from the amygdala, that are commonly involved in temporal lobe epilepsy [7].
Thus, the present study was designed to evaluate the hormonal profile of epileptic women with PCOS and to determine the impact of weight reduction regimen on their ovulatory function in comparison to non-epileptic PCOS women and normal fertile control women.
Patients and Methods
The present study was conducted at Departments of Neurology, Obstetrics & Gynecology and Clinical Pathology, Al-Dar Hospital, and Saudi German Hospital, Madinah, KSA. After obtaining written fully informed patients’ consents, the study included 60 PCOS diagnosed depending on the Rotterdam criteria, in which at least two of the following three criteria were met: Oligomenorrhea (<8 spontaneous menstrual cycles per year for at least 3 years before enrollment) or amenorrhea, biochemical hyperandrogenemia (serum total testosterone level >0.8 ng/ml), and polycystic ovaries (>12 follicles in the 2-9 mm range, thickened stroma and/or an ovarian volume >10 ml per ovary by vaginal ultrasound) [8,9].Among the enrolled PCOS women, 40 women were epileptic (Study group) and 20 women were non-epileptic as a positive control group for PCOS. The study also, included 20 normal non-epileptic fertile women of cross-matched age with normal ovulation and no signs of hyperandrogenemia with body mass index <24.9 kg/m2 as negative control group for both epilepsy and PCOS. Women had systemic or central nervous system illnesses (apart from epilepsy) that could interfere with hypothalamic-pituitary-ovarian function, receiving any other medications for any medical illnesses, hormonal or psychotropic medications apart from antiepileptic drugs, had current or previous pregnancy within 1 year of enrollment or on the use of hormonal contraception within the last 6 months prior to inclusion in the study were excluded.
The epileptic disorders were classified in accordance with the ILAE Classification [10] and treatment received prior to enrollment remains constant throughout the whole study period. In all patients transvaginal ultrasonographic (TVU) examination was performed within day 5 of the cycle at time of enrollment and anthropometric measurements including body height measured using a stadiometer (SECA, Hamburg, Germany), and weight was measured using electronic digital scales (Mercury, AMZ 14, Tokyo, Japan) were determined to calculate BMI as weight (kg)/height (m2) [11]. Obesity grades were defined after the WHO expert consultation [12] as BMI <24.9 as average, 25-<30 kg/m2 as overweight, BMI ≥30-35 kg/m2 as obese and BMI ≥35 kg/m2 as morbid obese.
Laboratory investigation
Blood samples were obtained in the early follicular phase of the basal cycle (days 5-7) in a plane container and were left to clot then serum was separated by centrifugation at 3000 rpm for 10 min. and stored at -20°C until analysis. Follicle stimulating hormone (FSH), leutinizing hotmone (LH), prolactin (PRL), 17ß-estradiol (E2), Testosterone (T) and sex-hormone binding globulin (SHBG) were measured by RIA using commercial enzymatic kits (Diagnostic Systems Laboratories). Progesterone levels ≥3 ng/ml on day 21 were considered indicative of ovulation.
Weight reduction program
All PCOS women were prescribed the same energy-restricted, high-protein diet (5000-6000 kJ/d) for a planned weight loss of 10-15 kg over the study period. The diet provided 30% of energy as protein, 40% as carbohydrate, and 30% as fat. In addition, women were assigned to undergo a walking/jogging program comprising five sessions per week. The training heart rate (HR) was based on a percentage of the maximum heart rate achieved in the treadmill tests conducted at time of enrollment and at 6 m.
Statistical analysis
Results were expressed as mean ± SD, range, numbers and percentages. Intra-group data was statistically analyzed using paired t-test and inter-group analysis was examined using Wilcoxone Ranked test for related data (Z test). Statistical analysis was conducted using SPSS statistical program, (Version 10, 2002). P value <0.05 was considered statistically significant.
Results
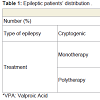
Neurological assessment defined 25 patients (62.5%) affected by idiopathic or cryptogenic generalized epilepsy and 15 patients (37.5%) were affected by symptomatic or cryptogenic localizationrelated (focal) epilepsy. Twenty-four (60%) were given antiepileptic drugs (S1 group); 14 patients were receiving monotherapy and 10 were receiving polytherapy, while 16 patients (40%) were untreated (S2 group), as they are following traditional therapy (Table 1).Studied patients showed non-significant difference as regards body weight, body height and body mass index; while all showed significantly higher body weight and body mass index compared to control group (Table 2).
Weight reduction regimen for 6-months resulted in significant reduction of body weight and BMI of PCOS women compared to their measures at the start of the regimen with non-significant difference among studied groups as regards both body weight and BMI determined at the end of the weight reduction regimen (Table 3).
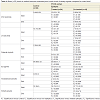
Baseline estimation of serum hormones profile showed discrepant results that were significantly deviated in all studied women from the control levels. However, the impact of epileptic therapy was evident and manifested as significantly (p<0.05) lower FSH, LH and prolactin in untreated women compared to controls, non-epileptic patients and epileptic women receiving treatment. On the other hand, antiepileptic therapy showed no impact on serum E2 or androgenic hormones manifested as non-significant (p>0.05) difference among studied patients with significant difference compared to control levels. The applied weight reduction regimen allowed significant reduction of serum androgens in treated women with epilepsy and non-epileptic PCOS women compared to their corresponding baseline levels. On the other hand, serum E2 showed significant (p<0.05) increase in nonepileptic, but was non-significantly (p>0.05) increased in patients with epilepsy compared to their corresponding baseline levels (Table 4).
Despite the promising results of combined weight reduction regimen and antiepileptic therapy, patients maintained on VPA showed the least results and showed significantly (p<0.05) higher serum FSH, prolactin and testosterone with significantly (p<0.05) lower serum E2, but non-significantly (p>0.05) higher SHBG compared to those receiving other forms of antiepileptic therapy (Table 5).
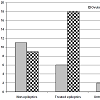
Throughout follow-up period, 19 women got ovulation as judged by serum progesterone >3 pg/ml and using TVU with success rate of 31.7%. Eleven women of non-epileptic women got ovulation with success rate of 55%. Eight patients with epilepsy had ovulation with a success rate of 20% among women with epilepsy; 6 women on antiepileptic therapy had ovulation with success rate of 25% and only 2 of women who were not treated had ovulation with a success rate of induction of ovulation among patients with epilepsy without treatment of 12.5% (Figure 1).
Discussion
The present study illustrated the impact of epilepsy with associated neurological disturbances on the feminine hormonal milieu as manifested by increased serum FSH, LH and prolactin in studied patients with epilepsy compared to control fertile women; a finding indicating the impact of epilepsy on the pituitary function either through disturbing the hypothalamic or higher centers control. These data are in line with Murialdo et al. who found that in epileptic patients; serum E2, free E2, and progesterone levels were lower in both ovarian phases, whereas those of SHBG were higher than in controls with no significant changes in hormone levels and in prevalence of anovulatory cycles between patients grouped according to their seizure frequency [13]. Galimberti et al. also, found serum E2, progesterone, and free estrogen index were lower, whereas SHBG levels were higher in the epilepsy patients than in the controls, but their levels were not different between groups of patients categorized according to seizure frequency score [14].Epileptic patients showed higher SHBG levels compared to nonepileptic women and those on treatment compared to untreated patients, a finding indicating a possible role of AEDs on the increased levels of SHBG and subsequent decreased free estrogen level that could be attributed to consumption by the high levels of SHBG. These data go in hand with Isojärvi who attributed the role of AEDs in induction and/or maintenance of feminine subfertility or infertility to the fact that the use of the liver enzyme inducing AEDs phenobarbital, phenytoin and carbamazepine increases serum SHBG concentrations in both men and women with epilepsy and over time the increase in serum SHBG levels leads to diminished bioactivity of testosterone and estradiol, which may result in diminished potency in men and menstrual disorders in some women, and, thus, to reduced fertility [15].
Differentially, AEDs induced varied disturbances in studied women hormonal milieu as patients maintained on VPA showed significantly higher serum FSH, prolactin and testosterone with significantly lower serum E2, but non-significantly higher SHBG compared to those receiving other forms of antiepileptic therapy. These data indicated a fact that VPA therapy allowed maintenance of the hyperandrogenic state and go in hand with Isojärvi who reported that valproate medication in women is associated with a frequent occurrence of reproductive endocrine disorders characterized by polycystic changes in the ovaries, high serum testosterone concentrations and menstrual disorders and young women with epilepsy seem to be especially vulnerable to the effects of VPA on serum androgen levels [15].
In support of the obtained data, Leskiewicz et al. reported that valproate decreased FSH-stimulated estradiol release and enhanced testosterone level, while carbamazepine enhanced SHBG concentration [16]. Verrotti et al. also, reported that AEDs may modulate hormone release from the hypothalamic-pituitarygonadal axis and they may alter the metabolism of sex hormones and their binding proteins; hepatic enzyme-inducing AEDs, such as carbamazepine and phenytoin, may be most clearly linked to altered metabolism of sex steroid hormones, but valproic acid, an enzyme inhibitor, has also been associated with a frequent occurrence of polycystic ovary syndrome and hyperandrogenism in women with epilepsy [5]. Drislane et al. stated that LH pulse frequencies in women with Temporal lobe epilepsy (TLE) may be influenced by the laterality of the epileptic focus, the reproductive endocrine status, and the use of antiseizure medications [17].
As regards the benefits gained by weight reduction program, nonepileptic PCOS patients showed more weight reduction compared to patients with epilepsy despite the difference was non-significant, however, all PCOS patients showed significantly lower body weight and BMI compared to their baseline data, but the extent of change was less in treated patients. The reported weight loss and changes in hormonal milieu in non-epileptic women indicated a role of dieting regimens and exercise in affecting the ovulatory functions of nonepileptic PCOS women. In support of this attribution, 11 out of 19 got ovulation were non-epileptic women and goes in hand with that reported in literature. Thomson et al. reported significant reductions in waist circumference, blood pressure, fasting insulin, fasting glucose, homeostasis model assessment of insulin resistance, testosterone, free androgen index, and an increase in SHBG with improved heart rate recovery which suggests improved autonomic function and thus highlights the importance of weight loss and exercise to reduce the cardiovascular disease risk in PCOS women [18]. Vause et al. defined the guidelines for management of PCOS as following: weight loss, exercise, and lifestyle modifications have been proven effective in restoring ovulatory cycles and achieving pregnancy in overweight women with PCOS and should be the first-line option for these women [19]. In support of the applied intervention model without medications, Karimzadeh & Javedani reported that lifestyle modification of PCOS women achieved a significant reduction in waist circumference, total androgen, and lipid profile with clinical pregnancy rate of 20% with lifestyle modification compared to 12.2% in those received clomiphene citrate alone, 14.4% with metformin alone, 14.8% with combined clomiphene citrate and metformin [20].
Despite the reported non-significant difference between treated and untreated epileptic PCOS women, women treated with AEDs showed non-significantly higher body weight and BMI and this could be attributed to the effects of AEDs on various endocrinal systems. In support of this attribution, Verrotti et al. stated that carbamazepine, oxcarbazepine or joined administration of carbamazepine and valproate decrease thyroxine (T4) level in patients with no effect on TSH, enhance leptin and insulin blood level and increased body weight [5].
The beneficial effect of maintenance therapy on fertility status despite these documented effects of differential lines of treatment was evident as 6 of the 8 patients with epilepsy got ovulated were on treatment but with polytherapy. This finding could be attributed to the in vitro findings of Killer et al. who found that in both, humans and cell lines, estrogen receptor alpha expression was increased by the P450-inducing AEDs, but not by prednisolone, which indicates that pathways different from CYP3A4/11 led to estrogen receptor alpha enhancement with subsequent induced estrogenic activity and commencement of feminine hormonal milieu and subsequently ovulation [21].
Thus, combined weight reduction program with antiepileptic therapy can break through the closed triangle of epilepsy, AED and hormonal disturbance resulting in anovulation and subsequently subfertility or even infertility. In support of such assumption, Moran et al. reported that the emerging evidence suggests that exercise offers additional benefits to dietary energy restriction for reproductive features of PCOS and should be used as the primary therapy in overweight and obese women with PCOS for the treatment of metabolic complications and improved ovulatory function and pregnancy [22].
It could be concluded that in epileptic PCOS women when polytherapy was provided with avoidance of VPA, combined weight reduction regimens associated with aerobic physical exercise intervention induce weight loss with subsequent amelioration of inhibitory effect of obesity on the reproductive function and adjustment of the hyperandrogenic milieu and induction of ovulatory cycles that promise for regaining fertility.
References
- Morrell MJ (1998) Effects of epilepsy on women’s reproductive health. Epilepsia 39: S32-S37.
- Jeanes YM, Barr S, Smith K, Hart KH (2009) Dietary management of women with polycystic ovary syndrome in the United Kingdom: the role of dietitians. J Hum Nutr Diet 22: 551-558.
- Pennell PB (2009) Hormonal aspects of epilepsy. Neurol Clin 27: 941-965.
- Silveira DC, Klein P, Ransil BJ, Liu Z, Hori A, et al. (2000) Lateral asymmetry in activation of hypothalamic neurons with unilateral amygdaloid seizures. Epilepsia 41: 34-41.
- Verrotti A, D’Egidio C, Coppola G, Parisi P, Chiarelli F (2009) Epilepsy, sex hormones and antiepileptic drugs in female patients. Expert Rev Neurother 9: 1803-1814.
- Luef G (2010) Hormonal alterations following seizures. Epilepsy Behav 19: 131-133.
- Quigg M, Kiely JM, Shneker B, Veldhuis JD, Bertram EH 3rd (2002) Interictal and postictal alterations of pulsatile secretions of luteinizing hormone in temporal lobe epilepsy in men. Ann Neurol 51: 559-566.
- Chen MJ, Yang WS, Yang JH, Hsiao CK, Yang YS, et al. (2006) Low sex hormone-binding globulin is associated with low high-density lipoprotein cholesterol and metabolic syndrome in women with PCOS. Hum Reprod 21: 2266-2271.
- Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, et al. (2007) Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49: 1442-1447.
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, et al. (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 51: 676-685.
- Khosla T, Lowe CR (1967) Indices of obesity derived from body weight and height. Br J Prev Soc Med 21: 121-128.
- WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157-163.
- Murialdo G, Magri F, Tamagno G, Ameri P, Camera A, et al. (2009) Seizure frequency and sex steroids in women with partial epilepsy on antiepileptic therapy. Epilepsia 50: 1920-1926.
- Galimberti CA, Magri F, Copello F, Arbasino C, Chytiris S, et al. (2009) Changes in sex steroid levels in women with epilepsy on treatment: relationship with antiepileptic therapies and seizure frequency. Epilepsia 50: 28-32.
- Isojärvi J (2008) Disorders of reproduction in patients with epilepsy: antiepileptic drug related mechanisms. Seizure 17: 111-119.
- Leskiewicz M, Budziszewska B, Lason W (2008) Endocrine effects of antiepileptic drugs. Przegl Lek 65: 795-798.
- Drislane FW, Coleman AE, Schomer DL, Ives J, Levesque LA, et al. (1994) Altered pulsatile secretion of leuteinizing hormone in women with epilepsy. Neurology 44: 306-310.
- Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, et al. (2010) Heart rate recovery improves after weight loss in overweight and obese women with polycystic ovary syndrome. Fertil Steril 93: 1173-1178.
- Vause TD, Cheung AP, Sierra S, Claman P, Graham J, et al. (2010) Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can 32: 495-502.
- Karimzadeh MA, Javedani M (2010) An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril 94: 216-220.
- Killer N, Hock M, Gehlhaus M, Capetian P, Knoth R, et al. (2009) Modulation of androgen and estrogen receptor expression by antiepileptic drugs and steroids in hippocampus of patients with temporal lobe epilepsy. Epilepsia 50: 1875-1890.
- Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ (2009) Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 92: 1966-1982.