Journal of Neurology and Psychology
Download PDF
Review Article
Sleep-Wake Disorders: Definition, Contexts and Neural Correlations
Giulio Perrotta*
Department of Criminal and Investigative Psychology Studies, Italy
*Address for Correspondence: Giulio Perrotta, Department of Criminal and Investigative Psychology Studies, University of Federiciana, Cosenza, Italy, Phone: (+39) 349 21 08 872; E-mail: giuliosr1984@hotmail.it
Submission: May 23, 2019
Accepted: July 05, 2019
Published: July 08, 2019
Copyright: © 2019 Perrotta G, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Starting from the macro-category “sleep-wake disorders”, as
defined in DSM-V, the individual pathological conditions were defined,
focusing on the contextual and clinical aspects, to continue the
analysis on neural correlates and strategic therapy to be used to solve
the problems encountered.
Keywords
Psychology; Neuroscience; Anxiety; Panic; Terror; Anxiety
disorders; Panic attack; Panic disorder; Nightmares; Sleepwalking;
Sex in sleep; Nocturnal paralysis; Hallucinations; Nocturnal awakenings;
Awakening; Narcolepsy; Hypersomnia; Amygdala; Prefrontal cortex;
Fear; Anxiety; Psychotherapy; Psychopharmacology; Benzodiazipines;
Antidepressants; Strategic approach
Introduction
Sleep: definition and contexts:
The sleep is defined as a state of rest opposed to waking. Various
definitions indicate sleep as “a periodic suspension of the state of
consciousness” [1,2], during which the body recovers energy; state of
physical and mental rest, characterized by the temporary detachment
of the conscience and the will, by the slowing down of the neuro
vegetative functions and by the partial interruption of the subject’s
sensorimotor relationships with the environment, indispensable for
the restoration of the organism” [3]. Like waking, in fact, sleep is an
active physiological process that involves the interaction of multiple
components of the central and autonomic nervous system. In fact,
although sleep is represented by an apparent state of rest, during this
state complex changes take place in the brain that cannot be explained
only as a simple state of physical and mental rest. For example, there
are some brain cells that at some stages of sleep have an activity 5-10
times greater than what they are awake [4].Two fundamental characteristics distinguish sleep from the
waking state: the first is that in sleep a perceptive barrier is created
between the conscious world and the external world, the second is
that a sensory stimulus of a certain level (for example a loud noise)
can overcome this barrier and make the sleeping person wake up.
Proper sleep is biologically imperative for sustaining life. The
psychophysical health of the individual depends on the quality and
duration of sleep. Sleep disorders, such as insomnia, are present
in many psychiatric disorders, in which sleep deprivation has a
significant impact on a person’s quality of life.
It is difficult to give a precise and unambiguous definition of
sleep. One of the mostaptis the one given in 1985 by Fagioli and
Salzarulo, who present it as “a state of the organism characterized
by reduced reactivity to environmental stimuli that involves a
suspension of relational activity (relationships with the environment)
and modifications of the state of consciousness: it is established
autonomously and periodically, is self-limited in time and is
reversible “.Another well accepted definition efinesitas “a temporary
and reversible detachment of the mind from the body, indispensable for the proper functioning of both” [5].
Yet another definition indicates it as: “A readily reversible state of
reduced activity and interaction with the surrounding environment.”
Thus the term “readily reversible” cannot be associated with coma or
anesthesia which, respectively, are pathology and a pharmacologically
induced state of rest.
Sleep therefore differs from other states of altered consciousness:
a) With sleep the abolition of the state of vigilance is, as already
mentioned, reversible. Thus the subject can awaken after an even
painless stimulus;b) Otherwise, stupor is an alteration of the state of consciousness
from which one can awaken only after administering a painful
stimulus;
c) The comatose state is an alteration of the state of consciousness
from which one cannot awaken after administering a painful stimulus;
d) Brain death is much more serious with the irreversible
cessation of all brain activity.
Traditionally, three main measures have been used to define sleep
physiology:
a) The electroencephalogram (conventionally abbreviated as
“EEG”) which translates brain activity into electrical waves;
b) The electrooculogram (conventionally abbreviated as “EOG”)
records eye movements and translates them into electric waves;
c) The electromyogram (conventionally abbreviated as “EMG”)
which records muscular activity (usually in polysomnography that
of the mylohyoid muscle). For a thorough examination of sleep we
use polysomnography, with which we record, during an entire night,
a series of physiological parameters, such as the movement of the
ribcage and the abdomen, the flow of air that passes through the
oronasal cavity, blood oxygen saturation and heart rate.
In 1953, Eugene Aserinsky and Nathaniel Kleitman discovered
the presence of Rapid Eye Movements (REM) during sleep. This
simple observation made it possible to differentiate sleep in a REM
phase (with rapid eye movements) and in a non-REM phase (NREM phase). In 1963, Kleitman and Dement described for the first time
the alternation, during the period of sleep, of REM and NREM
sleep in cycles, introducing the concept of sleep architecture. At
the end of the 1960s, after the discovery of REM and NREM sleep
and the concept of cyclical nature of these two phases within sleep,
the need arose to classify the electroencephalographic changes
that occurred during sleep in a macroscopic manner in a standard
manner. In 1968, Rechtschaffen and Kales based on the analysis of
electroencephalographic, electromyographic and electrooculographic
parameters classified sleep in 5 stages: 4 NREM stages (stage 1; stage
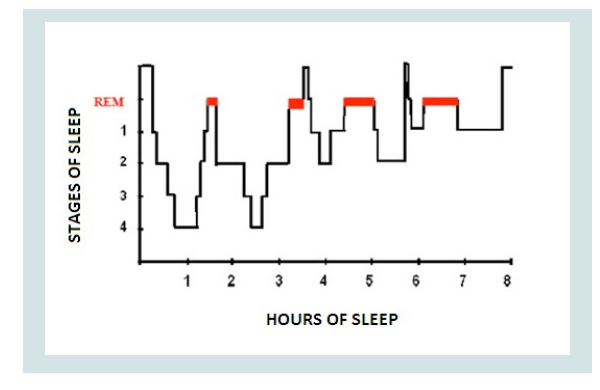
2; stage 3; stage 4) and a REM stage (Figure 1).
Figure 1: Hypnogram.
Source: https://www.researchgate.net/figure/Hypnogram-of-sleep-cycle-in-ahealthy-
young-adult-Normal-sleep-involves-cycling_fig1_267910844
Sleep presents a regular alternation of non-REM and REM phases
consisting of cycles of similar duration to each other. After falling
asleep the subject progressively passes from stage 1 of non-REM sleep
to stage 2, after which he passes to stage 3 or stage 4 and then, between
70 and 90 minutes after falling asleep, the first phase of REM sleep
occurs which lasts about 15 minutes. At the end of the first phase of
REM sleep the first cycle ends, which lasts approximately 80 to 100
minutes.
After the first cycle, others of a rather constant duration follow
one another, but where REM sleep tends to increase in duration at
the expense of non-REM sleep, in particular stages 3 and 4 (deep
sleep) which become shorter. During the night, in the end, REM sleep
constitutes about 25% of the total sleep duration. It is possible that
there are waking moments between the various cycles. The period of
sleep is represented graphically by the hypnograms that illustrate the
succession of the phases of wakefulness and sleep in relation to time.
Today in place of the subdivision into four stages the nomenclature in
three phases (N1, N2 and N3) adopted by the American Academy of
Sleep Medicine in 2007 on the basis of the appearance and frequencies
of the EEG oscillations, in which phase N3 combines stages 3 and 4
both characterized by the same large slow waves, even if in different
percentages.
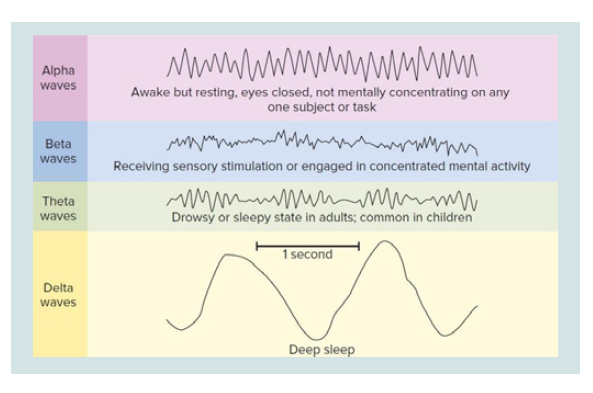
During the vigil, the EEG basically alternates between two
patterns. A pattern called “activation” (or desynchronized pattern)
characterized by low voltage waves (10-30 micro volts) and
high frequency (16-25 Hz) and a second called “alpha activity” characterized by sine waves of 8-12 Hz Alpha activity is typically
present and abundant when the subject is relaxed with eyes closed.
The activation pattern is present when the patient is in a state of
attention with open eyes. Eye movements are both rapid and slow
and muscle tone is medium to high.
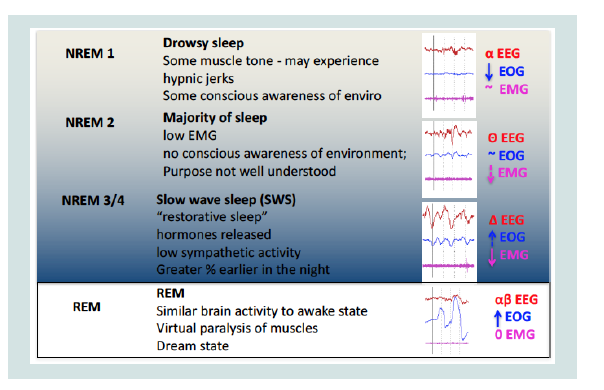
During stage 1, the alpha activity decreases, the activation pattern
is poor and the EEG consists mainly of low-voltage waves of mixed
frequency between 3-7 Hz. The movements of the eyes are still
present but slow, rotating and oscillators (not in phase opposition
as in the REM phase). The electromyogram shows a persistent tonic
activity although of a lower intensity than the wake. In stage 2 there is
a relatively low background activity, with variable frequency but close
to theta waves (3-7 Hz). Stage 2 is characterized by the presence of
two components, the so-called K complexes and the spindles of sleep
(or spindles). The latter of thalamic origin, lacking in lethal family
insomnia, a deadly disease for sleep deprivation. The eye movements
are slow, while the EMG is further reduced. In stage 3, 20% - 50% of
each epoch (conventionally an EEG recording period of 30 s) must
contain Delta activities, i.e., large amplitude EEG waves (> 75 micro
volts) and low frequency (about 0.5 - 4 Hz). The muscle tone in this
stage is slightly reduced and the eye movements practically absent.
The spindles of sleep may or may not occur, while the K complexes
are present, although they are often difficult to distinguish from delta
waves. Stage 4 is characterized by the presence of delta waves, which
here reach the maximum amplitude and minimum frequency, for
more than 50% of each era. As for stage 3, the spindles can be absent or
present while the K complexes are present, but almost unrecognizable
from the underlying delta rhythm. The movements of the eyes are
not present while a state of very low tonic muscle activation persists.
At this stage the metabolic activity of the brain is reduced (lower
consumption of oxygen and glucose). If the subject wakes up at this
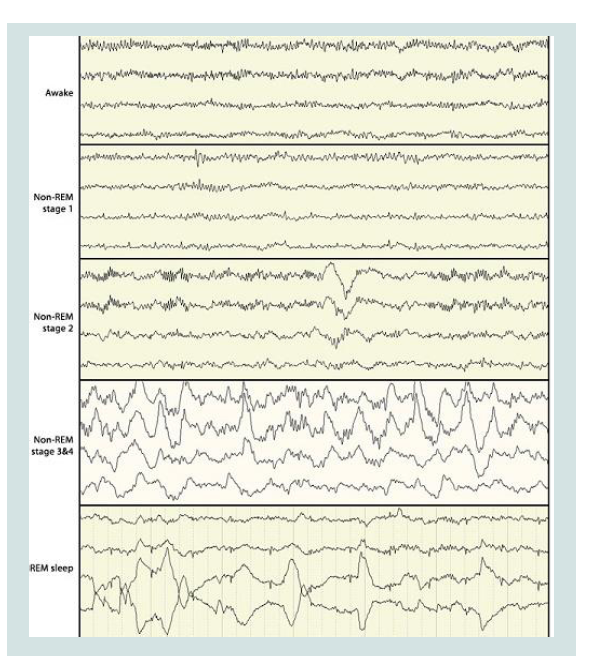
stage he can get confused for a few minutes (Figure 2-4).
The REM stage is characterized by a low voltage EEG with
mixed frequencies. The EEG of REM sleep is very reminiscent
of that of stage 1 if not for the discharged characteristics of waves
with the characteristic ‘saw-tooth’ morphology. PGO (ponto-genicoccipital)
waves appear, the activity of the hippocampus becomes
synchronized with the appearance of theta waves. The stadium takes
its name from rapid eye movements and the low tone of the mental muscles. Moreover, this phase is characteristic for the paralysis of
the muscles (to avoid mimicking dreams) and because it is the one
in which dreams predominantly occur. The brain consumes oxygen
and glucose as if the subject were awake and engaged in intellectual
activity. If you wake up in this phase you are perfectly oriented.
This stage is also characterized by a more imprecise control of the
vegetative functions of the organism, in fact the arterial pressure
increases and undergoes sudden changes, the heart rate increases and
extra systoles can appear, the respiratory frequency becomes more
irregular and the part is compromised thermo regulation. Penile
erection in men and genital changes in women may occur. REM sleep
tends to decrease with advancing age and reaches a peak at the age of
1 year and then decreases in favor of non-REM sleep.
Sleep deprivation was tested by Randy Gardner in 1965, a young
17-year-old student who stayed awake for 264 hours, or 11 days. On
the second day, his concentration diminished and subsequently lost
the ability to identify objects by touch. At the end of the third day
he experienced bad temper and disorientation. At the end of the
experiment it was difficult to concentrate, to remember recent events;
he became paranoid and began to hallucinate. On the eleventh day, the
medical personnel who kept him under control wrote: “Confusional
state and disorientation, sudden mood swings, irritability, speak with
a road sign believing him a man, hallucinations, temporary loss of
identity, difficulty in pronouncing tongue twisters, mumbles many
words, diminished reflexes, memory lapses, difficulty in focusing
objects, visual problems with too bright colors ... “. When we
sleep little, in fact, learning, memory, mood and quick reflexes are
compromised. Several studies show that sleeping systematically less
than six hours a night increases the risk of heart attack for well over four and a half times compared to those who sleep regularly.
Sleep-Wake Disorders: Classification and Clinical Contexts
When we talk about these disorders we refer to a wide range of
problems characterized by an alteration of the sleep-wake rhythm [6].
Those who present these disorders are unable to benefit from their
rest and perceive their sleep as insufficient or unsatisfactory in quality
and quantity, resulting in stress and discomfort during waking hours.
Due to the close connection between sleep quality and physical
and emotional health, even when a sleep disorder occurs occasionally
in a person, it should never be neglected. Sleep-related disorders are
accompanied by clinically significant distress or impairment in the
social, occupational or other important areas.
Sleep-wake disorders include the following sub-categories with related symptoms:
Insomnia disorder: predominant dissatisfaction with the
quantity or quality of sleep associated with difficulty in starting sleep
at bedtime, maintaining sleep, with frequent or prolonged awakenings
during the night and early morning awakening with difficulty go back
to sleep. For there to be a diagnosis of insomnia disorder the difficulty
of sleeping must occur at least 3 times a week and must persist for at
least three months despite adequate sleeping conditions.Hypersomnolence disorder: excessive sleepiness despite a main
sleep period of at least 7 hours, manifesting at least three times a week
for at least three months. It is accompanied by clinically significant
distress or impairment in cognitive, social, occupational or other
important areas. It is not attributable to the effects of a substance
(a substance of abuse, a drug) and the complaint of sleep inessis
not adequately explained by the coexistence of mental and clinical
disorders. It is not justified by another sleep disorder and does not
occur exclusively during the course of another sleep disorder.
Narcolepsy: It is characterized by recurrent periods of
irrepressible bi-dream of sleeping, sleep attacks or naps that occur on the same day. These episodes must have occurred at least three
times a week in the last 3 months. In order for there to be a diagnosis
of narcolepsy, episodes of cataplexy must occur at least a few times
a month, characterized by a sudden, brief and reversible episode of
muscle weakness that occurs in conjunction with emotional stimuli,
such as laughter, surprise, anger, joy or sadness.
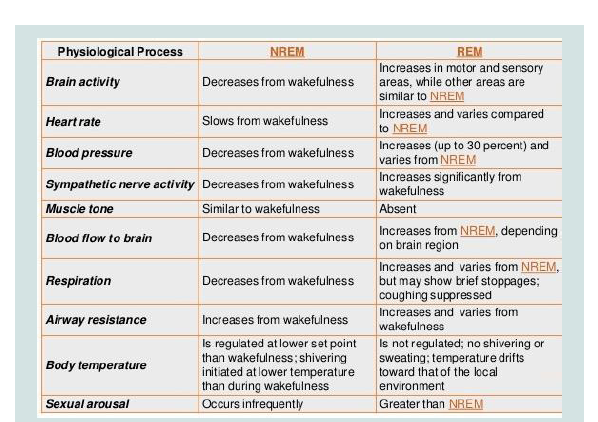
Figure 4: Sleepphisiology.
Part III. Source: https://www.slideshare.net/mabdelghani/physiology-ofsleep-
and-eeg-for-undergraduates
Respiratory-related sleep disorders:
a) Obstructive sleep apnea / hypopnea: repeated episodes of
obstruction of the upper airway (pharyngeal) during sleep that
can manifest itself with nocturnal respiratory disorders or daytime
sleepiness, asthenia or non-restorative sleep despite sufficient
opportunity to sleep and not explained by other mental disorder
or medical condition. Furthermore it is necessary that there are
polysomnographic evidences of 15 or more apnea and/or obstructive
hypopnea as per hour of sleep.b) Central sleep apnea: repeated episodes of apnea and hypopnea
during sleep caused by a change in respiratory effort. These are
ventilation control disorders in which respiratory events occur with
a periodic or intermittent pattern. This disorder is detectable when
polysomnographic evidences of 5 or more central apnea are present
per hour of sleep and when the disorder is not better explained by
another concomitant sleep disorder.
c) Sleep-relatedhypoventilation: polysomnography shows episodes of decreased respiration associated with high levels of carbon
dioxide, in the absence of a concomitant sleep disorder.
Circadian disorders of the wake-sleep rhythm: sleep interruption
due to an alteration of the circadian system, to a misalignment of the
endogenous circadian rhythm and the wake-sleep rhythm required
by the physical conditions of an individual or imposed by social
or work commitments. In these cases, sleep interruption leads to
excessive sleepiness or insomnia or both. Sleep disturbance causes
clinically significant distress or impairment in social, occupational or
other important areas.
Parasomnias: they are disorders characterized by abnormal
experiences and behaviors or physiological events that occur in
association with sleep, specific stages of sleep or sleep-wake passages.
According to the most recent classification of sleep disorders, parasomnias represent a large and heterogeneous group of sleep
disorders that consist of “undesirable manifestations that accompany
sleep and that often seem aimed at achieving a goal. In some cases they
can cause trauma and disturb sleep (of the patient or of those around
him)”. The different forms of parasomnia are classified according to
their occurrence during the different phases of sleep:
• NREM sleeps parasomnias (arousal disorders). NREM sleep,
especially during the deep sleep for this reason, these manifestations
occurs more frequently within 1-2 hours of falling asleep. An episode
on average last few minutes but its duration can be very variable: from a
few seconds up to even 30 minutes. Usually, NREM sleep parasomnias
arise in childhood (probably two to the high representation of deep
sleep during this phase of life) and tend to shrink or disappear with
adulthood. There is often familiarity with such episodes, which can
be triggered by certain factors such as sleep deprivation, irregular
sleep-wake cycles, fever, infections, alcohol, certain medications and
other sleep disorders including sleep apnea. Patient soften do not
retain any memory of the episodes themselves, the clinical features
of which can be very heterogeneous. We distinguish 3 different types
of manifestations (which can also occur in the same subject) which,
according to the most recent interpretations, represent a continuum
of the same phenomenon, with different degrees of complexity:
a) Confusion awake up. Episodes of partial wakening not
associated with walking or autonomic disorders (the child seems to
be awake but confused, disoriented, sometimes aggressive, does not
respond adequately to orders, can speak but not in an inconsistent
way);
b) Sleepwalking. Episodes characterized by more or less complex
automatic behavior (like walking, eating, drinking, and leaving home
...);
c) Night terrors. Episodes of partial wakening, often with sudden
onset, with expression of terror, intense agitation, sweating, pallor,
wheezing, tachycardia.
• Parasomnias usually associated with REM sleep
They are complex motor manifestations that occur during REM
sleep:
a) Behavioral disorder in REM:
REM sleep represents that phase of sleep, mostly represented in
the second part of the night, in which there is an almost complete
loss of tone of the voluntary musculature (“it is as if immobilized”)
and during which the more dreamlike activity occurs intense. The
behavioral disturbance in REM sleep (RBD) is characterized by
the loss of physiological muscle a tony. For this reason, during the
episodes, which occur more frequently in the second part of the night,
the patients present an excessive motor activity, often characterized
by abrupt behavior (such as screaming, punching and kicking), in
relation to the content of their dreams. In fact, patients often report
dreams with a negative content, which they “act” by performing
violent actions, which can assume characteristics of aggressiveness,
for example towards the bed partner. These manifestations therefore
entail a high risk of trauma both for the patient and for those close
to him. Their duration is usually between 2 and 10 minutes and the
frequency can be very varied: from weekly or monthly episodes to multi-night (4-5 / night).b) Sleep paralysis:
They consist in the inability to perform any voluntary motor
activity (one has the perception of being completely immobilized),
although the subject is completely conscious. They can occur during
the phase of falling asleep (“hypnagogic paralysis”) or following an
awakening (“hypnopompic paralysis”). They can be accompanied
by auditory or visual hallucinations and can last from a few seconds
to several minutes, often causing intense anxiety in the person who
lives them. They can be resolved spontaneously or following sensory
stimulation. They can be favored by an irregular sleep-wake rhythm
and sleep deprivation.c) Nightly nightly:
They consist of fearful dreams, with a negative content, often
of long duration; these dreams frequently induce the awakening of
the subject that keeps a vivid memory of it. They are common in
children or in patients with “post-traumatic stress disorder”. They
can be favored by fever or by the abrupt withdrawal of alcohol or
drugs that reduce REM sleep (amphetamines, some antidepressants
and benzodiazepines). These conditions, in fact, could lead to a sharp
and significant increase in the representation of REM sleep, favoring
the occurrence of nightmares.• Other parasomnias.
a) Groaning (Catathrenia):
It consists of the emission of a monotonous vocalization during
a prolonged exhalation associated with bradypnea (reduction of
respiratory rate). It often arises at a young age (20-30 years). The
episodes, which last about 2-20 seconds, occur more frequently during
REM sleep (especially in the second half of the night). The cause still
appears to be unknown and, at present, there is no treatment. Cases
have been described in association with sleep apnea and only a few
have been resolved with nocturnal ventilatory treatment. However,
it is important to distinguish such manifestations from snoring
episodes.b) Exploding head syndrome:
It is characterized by the perception of a sound often very intense,
similar to an explosion or an explosion, which occurs especially during
the phase of falling asleep, often resulting in a sudden wakening. The
perceived sound, although very violent, is never accompanied by
pain, but sometimes it can be related to visual flashes. If the crises
are recurrent and the sound perceived is particularly intense, the
patient will tend to fall asleep with fatigue for fear of a new attack.
The syndrome affects mainly women around the age of 50 and can
be favored by stressful conditions. Currently there is no univocal
explanation of these phenomena as well as an effective therapy (some
cases described have been treated with calcium channel blockers).c) Hypnagogical / Ipnopompical hallucinations.:
Vivid experiences, similar to dreams, often with bizarre or
terrifying contents, which occur during sleep (“hypnagogic”) or
after awakening (“hypnopompic”). During the attacks the fantastic
sensations can be mistaken for real. In most cases these are visual hallucinations, but they can also be auditory, tactile, gustatory or
olfactory. They may be associated with sleep paralysis and, like these,
occur frequently in individuals without other sleep disorders or may
be one of the symptoms of narcolepsy.d) Sleep-related eating disorder (SRED).:
Consists of repeated episodes during sleep of compulsive food
or drink ingestion (even unusual or inedible); often the level of
consciousness during the episodes appears to be null or partial, just as
the patient on waking frequently does not retain any memory of what
happened. This form of parasomnias appears more frequent in the
female sex and has an onset around the 20-30 years; familiarity with
other NREM sleep parasomnias is common. SRED can be associated
with other sleep disorders (arousal disorders, restless legs syndrome,
sleep apnea, narcolepsy) and can be triggered by taking certain drugs
(benzodiazepines, zolpidem, lithium) or by abrupt withdrawal of
alcohol intake. This syndrome must be distinguished from other
forms of eating behavior in sleep such as “Night Eating Syndrome”,
characterized by hyperphagia (overeating) evening and night and / or
anorexia (lack of appetite) morning and insomnia.The Neural Correlates in Sleep-Wake Disorders
A first system that controls and maintains the waking state is
represented by the aminergic nuclei of the brainstem [7], in particular
by the noradrenergic neurons of the locus coeruleus and by the
serotonergic neurons of the raphe nuclei, but it is assumed that the
substance dopaminergic neurons also play a role Black. These neurons
project diffusely to the cortex, the thalamus, the hypothalamus and
the hippocampus. When the subject is alert, the discharge frequency
of the neurons of these systems is maximum, is greatly reduced during
non-REM sleep and almost completely during REM sleep, suggesting
that they are systems involved in waking maintenance. These neurons
can also undergo phenomena of self-inhibition that promote sleep.
Conditions that stimulate activity promote wakefulness, but if these
systems are inhibited, sleep is promoted. If, however, it seems true that
the stimulation of the noradrenergic system stimulates and maintains
wakefulness, serotonin, while also stimulating wakefulness, favors,
over time, the synthesis and release of substances that promote sleep
and inhibit the cholinergic neurons of the forebrain basal, involved in
maintaining the vigil, thus playing an ambiguous role.
A second system that promotes wakefulness is the cholinergic
neurons of the basal forebrain. These neurons project to the cortex,
activating it, to the hippocampus and amygdale, and, in addition to
being awake; they are active during the REM phase, which are not very
active in the non-REM phase. They are inhibited by serotoninergic
terminations originating from the raphe nuclei. The cholinergic
nuclei of the brainstem include the laterodorsal nucleus of the
pontine tegmentum and the peduncolo pontine tegmentum nucleus
which are made up of two populations of neurons. A first population
is characterized by neurons active during REM sleep, which discharge
watery low frequency during wakefulness and non-REM sleep
and which project to the aminergic nuclei of the brainstem. The
second population consists of neurons whose discharge frequency
is maximum during wakefulness and during REM sleep and which
project to the thalamus and hypothalamus, activating them. The
tuberomammillary nucleus contains Histaminergic hypothalamicneurons which project diffusely to almost the entire central nervous
system, promoting waking maintenance and are maximally active
in this phase. The inhibition of these neurons with antihistamine
induces sleepiness. The posterolateral hypothalamus comprises a
small group of orexinergic neurons that maintain vigil and are also
involved in the regulation of food intake. They diffusely project to the
structures involved in the regulation of the sleep-wake cycle in the
central nervous system.
Vigil is a behavioral state characterized by arousal and cortical
activation, manifested in a desynchronized EEG pattern. In humans
it is characterized by a beta-type rhythm (frequency: 15 - 30 Hz;
amplitude: <20 μV) during active and alpha-type vigil (frequency: 8 -
12 Hz; amplitude: < 50 μV) during a relaxed vigil (Figure 5).
This behavioral state is supported by the interaction between
different brain regions and by different types of neuromedicators:
1) Glutamate (Glu). At the level of the brainstem, the Reticular
Formation (FR) is essential to maintain the typical activating
characteristics of waking. The FR is part of the Ascending Activating
System (ARAS) which through diffuse projections from the
brainstem reaches up to the cortex, causing desynchronization. The
projections that depart from the neurons present in the oral part of
the pontine and mesencephalic FR ascend towards the prosencephaly
and the cortex, where they sustain a cortical bone through a dorsal
pathway, towards the thalamus, and a ventral pathway, towards the
hypothalamus and the fore brain basal (Lindsley et al., 1950; Starzl et
al., 1951). On the other hand, the neurons present in the caudal part of
the pontine and bulbar FR facilitate the tone of the postural muscles
through their projections towards the motor neurons present in the
spinalcord (Jones, 2005). One of the neuromediators that is involved
in projections from FR nuclei is probably Glutamate (Glu) (Jones,
1995). The importance of glutamate in wakefulness is also underlined
by the fact that most anesthetics (including those for inhalation
and ketamine) attenuate glutamate-mediated neuro transmissions
(Rudolph and Antkowiak, 2004).
2) Nore Pinephrine (NA). The Locus Coeruleus (LC) is another
important structure for waking: its noradrenergic neurons stimulate
cortical activation and arousal through their diffuse projections to
the forebrain, the trunk of the brain and the spinalcord. The neurons
of the LC, in fact, discharge every frequently during wakefulness
(especially during active wakefulness), decrease their discharge
during slow-wave sleep and cease their activity during REM sleep
(Aston-Jones and Bloom, 1981; McCarley and Hobson, 1975). The
role of Nor Adrenaline (NA) is however ambivalent, as it depends
on the type of receptor on which it is going to act; in general, α-1
adrenergic receptors have an excitatory action (depolarization due
to the closure of potassium channels); on α-2 adrenergic receptors,
it has an inhibitory action (hyper polarization due to opening of
the potassium channels). Through its receptors, the NA selectively
excites the other systems that are involved in waking and inhibits
those structures that support sleep, especially at the level of the basal
prosencephalon and the preoptic area (Jones, 2005).
3) Dopamine (DA). At the most rostral level, in the mesencephalic
region, the Substantia Nigra (SN), the ventral tegmental area (VTA)
and the ventral Periaqueductal Gray (vPAG) play a priority role in attentional processes and in maintaining arousal, through direct
dopaminergic connections towards the striatum nucleus, the basal
forebrain and the cortex (Lu et al., 2006). These dopaminergic
neurons show the highest level of activity during wakefulness and
REM sleep, 33 while their discharge decreases during slow-wave sleep
(Lena et al., 2005; Maloney et al., 2002).
4) Serotonin (5-HT). The waking state is also maintained by the
neurons of other brain stem structures: the Dorsal Nucleus of the
Raphe (DR) and the Medial Nucleus of the Raphe (MR). The neurons
of these structures use serotonin (5-HT) as a neurotransmitter
and project towards many regions of the diencephalon, the limbic
system and the neocortex. These structures reach their maximum
peak discharge during wakefulness, decrease their activation during
NREM sleep and are silent during REM sleep.
5) Acetylcholine (Ach). Ponto-mesencephalic cholinergic
structures, such as the nucleus of the Laterodorsal tegmentum
(LDT) and the nucleus of the Pedunculopontine Tegmentum
(PPT), play an important role in maintaining wakefulness, but also
in REM sleep, facilitating arousal cortical and desynchronization.
Both structures, parallel to the neurons of the FR, project towards
the specific thalamus-cortical projection system, where they cause a
diffuse cortical activation (Jones, 1995; McCormick, 1992; Steriade et
al., 1990). In small part, these structures also project to the posterior
hypothalamus, to the basal pros encephalon and to the FR through the
extra-thalamic pathway. The discharge activity of these cholinergic
neurons of LDT and PPT is high during wakefulness, decreases
during NREM sleep and increases again during REM sleep (elMansari
M. et al., 1989; Steriade et al., 1990). The discharge of this cholinergic
system occurs in association with states of cortical activation (Jones,
2005; Steriade et al., 1990), but it is not related to behavioral arousal.
In fact, it has been shown that the injection at the level of the pontomesencephalic
tegmentum of Acetyl Choline Agonists (ACh), such
as carbachol, causes cortical activation accompanied by inhibition
of muscle tone (Jones, 2004b). According to this, ACh can act in
different populations of target cells to promote cortical activation
and inhibit muscle tone. In the thalamus, for example, ACh acts on
nicotinic receptors (nAChRs) and muscarinic receptors (M1ACh
and M2ACh) to facilitate cortical activation (Curro et al., 1991;
McCormick, 1992); in fact, Ach activates the thalamus-cortical relais
nuclei through an excitatory action directed on the nAChRs and
M1ACh receptors and through an indirect facilitation: inhibitory
action on the thalamic-reticular GABAergic neurons through the
M2ACh receptors. Similarly, in the FR of the brain stem, ACh can
act on different receptors to excite some neurons that are involved
it her in cortical activation or motor inhibition, and based on two
behavioral states, the wake and REM sleep, which are similar to each
other due to the desynchronization of the tracing, but differ in other
aspects, such as the presence or absence of muscular tony. How can we
explain this phenomenon? Under normal conditions there is a certain
balance between the noradrenergic and cholinergic systems so that
the activation of both neuronal systems maintains the waking state,
characterized by activation of the motor system and cortical arousal.
REM sleep and loss of muscle tone occur when the noradrenergic
system is inactive, while cholinergic is active. This association became
evident in the 1970s in an animal model through the administration
of Acetyl Cholinesterase Inhibitors (AChE), used to strengthen the activity of Ach. When they were administered alone, AchE inhibitors
stimulated wakefulness; conversely, when they were administered
following the removal of catecholamines (therefore also of NA), by
administration of reserpine, they stimulated REM sleep (Curro et al.,
1991; Jones, 2004b; McCormick, 1992). In men, acetyl cholinesterase
inhibitors, when given during waking, stimulate cortical activation
and induce prolongation of waking state; while, they accelerate the
onset of REM sleep when administered during NREM sleep, when
the noradrenergic system and other arousal systems are inactive
(Gillin and Sitaram, 1984). On a more rostra level, in the basal
pros encephalon, there are other neural compartments that use
acetylcholine as a neuro mediator, such as the medial septum (MS
/ vDB: medial septum / vertical limb of the diagonal band) and
other nuclei, which play a major role in cortical activation, receiving
input from structures of the brainstem and the hypothalamus, and
projecting diffusely towards the cortex (Jones, 2004a; Lee et al., 2005).
These neurons are very important in generating the theta rhythm (4 -
7 Hz) and gamma (30-60 Hz) during wakefulness and REM sleep. At
this level there are also other GABAergic and glutamatergic neurons
that increase their discharge in association with corticalactivation
(Gritti et al., 1997; Manns et al., 2003) and seem, above all the latter,
to be responsible for the increase in behavioral arousal and tone of
postural muscles (Gritti et al., 1994; Henny and Jones, 2006).
6) Histamine (His). As already observed by von Economo (von
Economo, 1930), another important region for waking maintenance
is the hypothalamus. The back and side of this structure appears
to have a specific role in cortical activation. The neurons of the
tuberomammillary nuclei (TMN), forming part of the posterior
rhypothalamus, use histamine as mediator and stimulate, through
diffuse projections, cortical activation (Brown et al., 2001b; Saper
et al., 2001). Histaminergic neurons discharge profusely during
wakefulness, decrease during NREM sleep and cease their activity
in REM sleep. Histamine has an excitatory effect on most of the
ARAS nuclei and, in contrast, inhibits the “sleep active” neurons of
the Ventro Lateral Preoptic area (VLPO), by excitatory synapses on
inhibitory inter neurons (Liu et al., 2010).
7) Oressin / Hypocretin (Orx / Hcrt).Finally, at the level of the
lateral hypothalamus (Lateral Hypothalamus, LH) there are neurons
that use a known peptide with the double word: oressin / hypocretinas
mediator. This plays a fundamental role in promoting and stabilizing
the waking state, while suppressing REM sleep. A deficiency of this
peptide leads to narcolepsy (Chemelli et al., 1999; Lin et al., 1999;
Mignot et al., 2002; Peyron et al., 2000), ie a neurological disorder
characterized by daytime sleepiness accompanied by cataplexy
(sudden loss of muscle tone), hypnagogic hallucinations (auditory and
/ or visual hallucinations during the phases of sleep / wake or sleep)
and sleep paralysis (inability to move or speak during awakenings)
(Yoss and Daly, 1957). Oressinergic neurons have connections with
all the nodes that intervene in the wake-sleep cycle and are actively
inhibited during the NREM sleep phase by the GABAergic neurons
of the preoptic region and of the basal pros encephalon. These
oressinergic nuclei maintain the vigil by a diffuse projection towards
the aminergic systems, in particular towards the LC, where they
promote the vigilat the expense of REM sleep (Bourgin et al., 2000;
Hagan et al., 1999). The anatomical distribution of the connections
of oressinergic neurons and their involvement in narcolepsy has led to the hypothesis that orexin, besides being important for waking
maintenance, is fundamental for the stabilization of the wake-sleep
switch (Saper et al., 2001).
Melatonin: Contexts and Clinical Profiles:
From a neurobiochemical point of view, as already stated [8],
the pineal gland produces melatonin, a hormone isolated for the first
time in 1958 by Aaron Lerner and produced by pinealocytes starting
from the neurotransmitter serotonin (5-hydroxy-tryptamine) for
N-acetylation and oxy-methylation, by virtue of the fact that these
cells contain the enzyme Hydroxyindole-O’xymethyltransferase
(HIOMT), epiphysis marker enzyme.It acts in the circadian rhythm of sleep and has powerful
antioxidant effects: melatonin is synthesized in the absence of
light from the pineal gland; shortly after the onset of darkness, its
concentrations in the blood increase rapidly and reach the maximum
between 2 and 4 am and then gradually decrease as the morning
approaches.
Exposure to light (especially at the blue wave length between
460 and 480 nm) inhibits the production of melatonin in a dosedependent
manner. It is therefore used for the short-term treatment
of insomnia over 55 years of age.
The side effects of melatonin are not null, although the contrary
belief is widespread: over the years, various professional bodybuilders
and various sports information magazines have affirmed the
possibility, with the support of some scientific studies, that daily
doses between 0.5 mg and 3 mg, taken 30-60 minutes before training,
increase the levels of growth hormone, without giving side effects,
which are usually recognized in irritability and drowsiness.
Melatonin decreases the release of GnRH: for this reason the
synthesis of testosterone and therefore libido decreases. More
precisely, it inhibits the secretion of the luteinizing hormone, which
stimulates the male endocrine activity of the interstitial cells of the
testis with testosterone and sperm production, and in the female
ovulation and conversion of the ovarian follicle into the corpus
luteum. Taken for prolonged periods, melatonin can have a depressive
effect in predisposed subjects; furthermore, it can inhibit ovulation
precisely because of the suppression of the GnRH release it causes.
Recent research in the biomedical field, especially on melatonin,
has shown that:
1) Melatonin is importantly involved in inflammatory processes
and cellular apoptosis [9].
2) Exposure to electromagnetic fields decreases the secretion of
melatonin [10], which negatively affects cellular processes linked to
death, acts on sex hormones and connected glands and interferes
with the sleep-wake rhythm [11].
3) Melatonin intervenes in the neurobiological processes involved
in anorexic and bulimic disorders and in the predisposition to be
subject to these psychophysical pathologies [12].
4) Melatonin is involved with cortisol in the immunomodulatory
response [13].
5) Melanin intervenes in the regulation of acidosis in malignant
tumor processes [14].
6) Melatonin is a potent inhibitor of ovarian and prostate cancer
[15,16].
7) Melatonin has positive effects on blood pressure, reducing
hypertension [17].
8) Melatonin, having antioxidant and modulating properties
of the circadian rhythm, has positive effects on drug therapy in the
presence of schizophrenia and in general in psychotic syndromes
[18,19].
9) Melatonin has a positive effect on blood sugar, reducing blood
levels and favoring a positive prognosis on insulinic therapy in the
rats [20].
10) Melatonin, compared to problems related to the central
nervous system, seems to be directly involved in the reduction of
tissue and nerve lesions, affecting free radicals due to its powerful
antioxidant effect. Since endogenous melatonin levels decrease
significantly in senility, these result simply that the loss of this
antioxidant could contribute to the incidence or severity of some agerelated
neurodegenerative diseases [21].
11) Melatonin, precisely because it is related to serum and ionized
calcium levels [22], positively intervenes in vertebral disorders
and intervertebral degeneration (in chicken) [23,24]; also in the
cartilaginous problems and curvature of scoliosis [25,26].
12) Melatonin has beneficial effects on respiratory problems
linked to obstructive bronchospasm [27].
In recent decades [28], the integration of melatonin in the diet
has allowed a significant and positive management of primary sleep
disorders, especially at a dose of 2-10 mg, unlike the classic 0,5-1 mg
dose indicated for jet lag disorder.
Conclusion
Sleep disorders represent a primitive alteration of its regulatory
mechanisms and therefore of the physiological rhythms of the sleepwake
cycle or be the clinical expression of other pathologies, organic
or psychic. Chronic sleep deprivation, altering the sleep-wake cycle
e all the associated biological rhythms, involves the exhaustion
of energy that feeds our life, compromising its quality in all its
dimensions: personal, affective, family, socio-relational and working.
Sleep is therefore a cornerstone of health.
For this reason, morphological disorders must always be taken
seriously consideration and shared with your doctor to be able to
identify and therefore eliminate, where possible, any causes triggers,
through a targeted behavioral and / or intervention pharmacological.
The so-called “sleep hygiene”, which is achieved through the adoption
of behaviors that aim to promote a good night’s sleep, already helps
improve sleep quality and maintain it over time, representing the
primary strategy.
The basic advice, in the absence of diseases, to sleep properly are:
- Respect your own sleep-wake rhythm, lying down and waking
up around the same time (even during the weekend) and avoiding naps during the day.
- Always follow the same ritual before going to bed, dedicating
yourself to relaxing activities.
- Perform physical activity during the day and not in the evening
hours.
- Take meals at regular times, preferring a light diet for dinner.
- Avoid the intake of exciting substances (tea, coffee, alcohol,
nicotine) in the evening hours.
- Rest on a comfortable bed in a cool, dark room silent and well
ventilated.
In the presence of a pathological disorder, however, the
intervention of a specialist in neurology or psychiatry is required to
carry out a targeted therapy consisting of pharmacological and/or
psychotherapeutic treatment.