Journal of Neurology and Psychology
Download PDF
Research Article
Astrocyte Activation is A Potential Mechanism Underlying Depressed Mood and Apathy in People with HIV
Ronald J. Ellis1*, Yan Fan2, David Grelotti3, Bin Tang3, Scott Letendre4 and Johnny J. He5
1Departments of Neurosciences and Psychiatry, University of
California, San Diego, CA, United States
2Department of Ophthalmology, UT Southwestern Medical Center,
Dallas TX, United States
3Department of Psychiatry, University of California, San Diego, CA,
United States
4Departments of Medicine and Psychiatry, University of California,
San Diego, CA, United States
5Department of Microbiology and Immunology, Chicago Medical
School Rosalind Franklin University, North Chicago, IL, United
States
*Address for Correspondence: Ronald J. Ellis, UCSD HNRC, 220 Dickinson Street Mail Code 8231, Suite B
San Diego CA 92103-8231 E-mail: roellis@health.ucsd.edu
Submission: November 18, 2022
Accepted: December 20, 2022
Published: December 26, 2022
Copyright: © 2022 Ellis RJ, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Background: Astrocytes become activated with certain
infections, and this might alter the brain to trigger or worsen depressed
mood. Indeed, astrocytes are chronically activated in people with
HIV infection (PWH), who are much more frequently depressed than
people without HIV (PWoH). A particularly disabling component of
depression in PWH is apathy, a loss of interest, motivation, emotion,
and goal-directed behavior. We tested the hypothesis that depression
and apathy in PWH would be associated with higher levels of a
biomarker of astrocyte activation, glial fibrillary acidic protein (GFAP),
in cerebrospinal fluid (CSF).
Methods: We evaluated PWH in a prospective observational
study using the Beck Depression Inventory-II (BDI-II) and additional
standardized assessments, including lumbar puncture. We measured
GFAP in CSF with a customized direct sandwich ELISA method. Data
were analyzed using ANOVA and multivariable regression.
Results: Participants were 212 PWH, mean (SD) age 40.9±9.14
years, median (IQR) nadir and current CD4 199 (57, 326) and 411
(259, 579), 65.1% on ART, 67.3% virally suppressed. Higher CSF GFAP
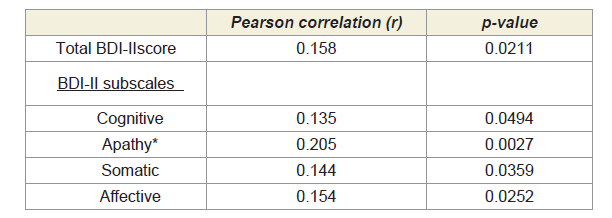
correlated with worse total BDI-II total scores (Pearson correlation
r=0.158, p-value=0.0211), and with worse apathy scores (r=0.205,
p=0.0027). The correlation between apathy/depression and GFAP was
not influenced by other factors such as age or HIV suppression status.
Conclusions: Astrocyte activation, reflected in higher levels of
CSF GFAP, was associated with worse depression and apathy in PWH.
Interventions to reduce astrocyte activation -- for example, using a
peptide-1 receptor (GLP-1R) agonist -- might be studied to evaluate
their impact on disabling depression in PWH.
Introduction
Despite viral suppression on combination antiretroviral therapy
(ART), people with HIV (PWH) suffer from a higher prevalence
of depression than the general population. Depression is the most
common psychiatric comorbidity in HIV [1] and apathy – a lack
of interest, motivation, emotion, and goal-directed behavior – is a
particularly prominent and frequent manifestation of depression
in PWH [4]. While often related to depression, apathy also occurs
in other brain disorders where dopaminergic neurotransmission
is disrupted, such as abulia and akinetic mutism. Dopaminergic
dysfunction is also common in PWH [5]. PWH who have depression
and apathy show poorer medication adherence [6], lower rates
of viral suppression [7,8] poorer quality of life [9,10], and shorter
survival [11-14]. Chronic systemic and neuro-inflammation persist
in virally suppressed PWH and predict morbidity and mortality
[15]. Apathy and anhedonia are linked to inflammation [16] as
evidenced by elevated levels of interleukin-(IL-)6 and tumor necrosis
factor (TNF)-α [17-22]. Inflammation, anhedonia, and apathy often
signal resistance to traditional antidepressants [23-29]. In the brain,
activated astrocytes mediate many aspects of immune function and
inflammation. Astrocyte activation is an important contributor to neuronal-glial network dysfunction in depression [30,31], as
would be expected based on the central role of astrocytes in brain
metabolism and inflammatory signaling. Astrocyte activation at
autopsy is associated with antemortem depressed mood [32,33].
Expression of glial fibrillary acidic protein (GFAP) is upregulated
in activated astrocytes [34], and cerebrospinal fluid (CSF) levels
of GFAP, a marker of astrocyte activation, are increased in people
with depression [35]. Astrocyte activation is a prominent feature of
brain disease in HIV and correlates with the release of neurotoxicviral
proteins such as Tat [36-41]. Although apathy is a particularly
prominent and disabling component of depression in PWH and
HIV causes astrocyte activation, no previous study has evaluated
CSF GFAP levels in PWH in the setting of depression and apathy .
We tested the hypothesis that elevated CSF GFAP levels, reflecting
astrocyte activation, would be correlated with depressed mood and
apathy in PWH.
Methods
This cross-sectional study evaluated PWH at 6 US centers in
CNS AntiRetroviral Effects Research (CHARTER), a prospective
longitudinal cohort, between 2003-2008. Inclusion criteria were
HIV seropositivity and ability to complete the protocol. Participants
who had severe neuropsychiatric comorbidities (e.g., untreated
schizophrenia or seizure disorder) were excluded. All study
procedures were approved by local Institutional Review Boards and
all participants provided written informed consent for the study
procedures, including future use of data and biospecimens.
All participants were comprehensively evaluated with
standardized assessments including lumbar puncture, phlebotomy,
neuromedical history and examination, and laboratory testing. A trained clinical examiner interviewed and examined participants to
collect information such as antiretroviral treatments, nadir CD4+ T
cell counts, and history of diabetes mellitus.
Depressive symptoms were assessed using the Beck Depression
Inventory (BDI-II), a validated survey of 21 questions that assess
depressive symptoms and their severity [42]. Higher BDI values
indicate higher severity depressive symptoms with a value >13
indicating at least mild depression. The BDI-II includes three standard
subscales capturing cognitive, somatic, and affective symptoms
of depression. Since we predicted that the apathy component
of depressed mood would be particularly important in HIV, we
constructed an apathy subscale using items that specifically address
apathy symptoms: loss of pleasure, loss of interest, indecisiveness,
and tiredness or fatigue (range 0-5, higher scores indicate worse
atrophy).Dependence in instrumental activities of daily living
(IADLs) was assessed using a modified version of the Lawton and
Brody Scale [43] that asks participants to rate their current and best
lifetime levels of independence for 13 major IADLs such as shopping,
financial management, transportation, and medication management
[9]. Individuals who reported difficulties in completing >2 IADLs
were considered functionally dependent.
Clinical Laboratory Evaluations:
HIV infection was diagnosed using an enzyme-linked
immunosorbent assay with Western blot confirmation. HIV RNA
in plasma was measured using commercial assays and deemed
undetectable at a lower limit of quantification (LLQ) of 50 copies/mL.
CD4+ T lymphocytes were measured by flow cytometry and nadir
CD4+ T lymphocyte count was assessed by self-report.CSF GFAP in picograms per milliliter (pg/mL)was measured
by a customized direct sandwich ELISA method, with a mouse
monoclonal antibody cocktail against GFAP (Covance, Cat#SM1-
26R) as the capturing antibody and a rabbit polyclonal anti-
GFAP antibody (DAKO, Cat# Z0334) as the detection antibody.
GFAP protein standards (Calbiochem, Cat# 345996) were used to
standardize concentration curves.
Statistical Analyses:
Demographic and clinical characteristics were summarized using
means and standard deviations, medians, and interquartile ranges
or percentages, as appropriate. Log10 transformation was used to
normalize CSF GFAP values. The Pearson correlation coefficient
was used to measure the relationship of GFAPlevels to indices
of depressed mood and apathy. We applied ANOVA when the
distribution of the outcome variable was not significantly different
from normal. When distributions significantly deviated from normal,
non-parametric analyses were conducted. Follow-up analyses used
recursive partitioning to identify informative GFAP cut-offs. We
adjusted for testing multiple related outcomes using the Benjamini
Hochberg procedure. When potential statistically confounding
variables such as age and demographic and disease variables were
significantly related to both the predictor (CSF GFAP level) and
outcomes of interest (apathy, depression), we evaluated these further
in multivariable regression analyses. Relevant covariates considered
included demographics, HIV disease and treatment parameters, and
antidepressant treatments. Analyses were conducted using JMP Pro version 15.0.0 (SAS Institute, Cary, NC, 2018).Results
The sample included 212 participants with a mean±SD age
40.9±9.14 years, female17.9%, black40.6%, non-Hispanic white
47.6%,Hispanic 8.96%, other race/ethnicity 2.83%, non-Hispanic
white 47.6%, median (IQR) duration of HIV infection 7.3 (2.58,
12.8) years, current CD4 411 (259, 579), nadir CD4199 (57, 326),
plasma HIV RNA suppressed (<50 copies/mL) in32.7%, CSF HIV
RNA suppressed in 62.3%. The mean±
SD log10 CSF GFAP level was
3.47±0.0781pg/mL, and the mean BDI-II score was 12.2±9.84, with
39.2% having a BDI-II>13, reflecting at least mild depression.
Potential statistical confounds:
Demographics Several variables were significantly related to
depression parameters and GFAP levels. Older individuals had both
worse apathy (r=0.220, p=0.0013) and higher log10CSF GFAP (r=0.357,
p=9.00e-8). In a multivariable regression predicting apathy scores,
the interaction between age and GFAP was not significant(p=0.888),
while both main effects of both GFAP (p=0.0442) and age (p=0.0197)
were significant. Apathy scores were not related to sex or ethnicity.
Older PWH also had worse overall depressed mood (BDI-II total
score; r=0.155, p=0.0305). In a multivariable regression predicting
BDI-II total score, the main effect of age (p=0.164) and the interaction
of age with GFAP (p=0.846) were not significant.Sex and ethnicity were not significantly related to BDI-II total
score. However, both sex and ethnicity were related to CSF GFAP.
Males had higher GFAP levels than females (mean±SD 3.48±0.0793
versus 3.44±0.0635, p=0.0033) and whites had higher levels than the
other ethnicities (non-Hispanic white [3.49±0.081] versus [black,
3.46±0.076] versus Hispanic [3.44±0.060] versus other race/ethnicities
[3.450.031]; p=0.0051). In a multivariable regression predicting BDIII
total score, GFAP was statistically significant (p=0.0165), while sex
and the interaction term were not(ps>0.25).In a similar regression for
ethnicity, GFAP was significant (0.0239), while ethnicity(p=0.237)
and the interaction (p=0.891) were not.
Antidepressant medications The proportion of participants
taking antidepressant medications was 32%. The odds of taking
antidepressants for those with a BDI-II>13 was 2.50 (95% confidence
interval 1.38, 4.54). Antidepressant use was associated with worse
apathy scores (3.45±2.34 versus 1.94±2.11, p=5.43e-6) and higher
GFAP levels (3.46±0.0789 versus 3.50±0.0706, p=0.0003).PWH
both on and off antidepressant medications contributed to the
relationship between GFAP and apathy: for those on at least one
antidepressant: r=0.123, p=0.295, N=67; for those not on any
antidepressants: r=0.139, p=0.0988, N=142;interaction p=0.895.
Laboratory Parameters PWH with detectable plasma viral
loads had worse total BDI-II scores (13.2±9.93 versus 10.2±9.43,
p=0.0409)as well as worse scores on the cognitive (4.82±4.76 versus
2.94±3.86, p=0.0049), but not apathy, somatic or affective items
(ps=0.0990, 0.3893 and 0.0856, respectively). Both virally suppressed
and unsuppressed PWH contributed to the relationship between
GFAP and apathy: for suppressed PWH r=0.126, p=0.302, N=69;
for not suppressed r=0.262, p=0.0016, N=142; interaction p=0.388.
Detectable viral load was not associated with CSF GFAP(3.47±0.0797 versus 3.48±0.0748, p=0.160). In a multivariable regression
predicting BDI-II cognitive scores, the main effects of both GFAP
(p=0.00139) and detectable viral load were significant (p=0.00316),
but their interaction was not (p=0.438). Findings were similar for
the BDI-II total score (data not shown). Those with suppressed CSF
HIV RNA had better apathy scores (2.17±2.26 versus 2.85±2.29,
p=0.0347). Higher GFAP correlated with higher CSF total protein
(r=0.310, p=4.51e-6), but CSF protein did not relate to apathy scores
(r=0.092, p=0.183). GFAP was not influenced by CSF leukocyte count
(r=-0.0136, p=0.845). Apathy scores did not correlate with current
(r=0.0353, p=0.610) or nadir CD4+ T cells (r=0.00936, p=0.893), or
plasma viral suppression (suppressed 2.058±2.60 versus unsuppressed
2.61±2.99, p=0.0990) (Table 1).
Table 1: Higher CSF GFAP levels (greater astrocyte activation) correlated with
worse Beck Depression Inventory-II (BDI-II) total and subscale scores (higher =
worse depression).
In a multivariable regression predicting BDI-II, GFAP was
significant while being on an antidepressant and its interaction with
GFAP were not (ps=0.153 and 0.359). In a stepwise multivariable
regression (p to enter 0.05, p to leave 0.05) predicting BDI-II total
score from CSF GFAP, age, sex, ethnicity, nadir, and current CD4+
T-cell count and viral suppression, the model selected CSF GFAP
(p=0.00915) and lack of viral suppression(p=0.00998) as the best
correlates (overall model p=0.0046).
Impact of Depression on Activities of Daily Living and Quality of Life: PWH with worse depression (higher BDI-II scores) had worse
HIV-MOS physical health summary scores (r=-0.626, p=1.85e-23),
and worse mental health summary scores (r=-0.825, p=1.25e-51).
Similarly, higher CSF GFAP correlated with worse physical (r=-0.177,
p=0.0116)and mental (-0.196, 0.0052) health scores. The proportion of
participants reporting dependence in instrumental activities of daily
living (IADLs) was 15.6%; participants with a BDI-II>13 had 11.9-
fold higher odds of being dependent (95% CI 4.68, 36.8; p=1.57e-8).
There was a 3% increase in the odds of having detectable plasma viral
load per one-unit increase in BDI-II scores increased (OR 1.03 [95%
CI 1.00, 1.07] per 1-point increase in BDI-II,p=0.0372). Similarly,
the odds of having detectable CSF viral load increased as BDI-II
scores increased (OR 1.03 [1.01, 1.06] per 1-unit increase in BDI-II,
p=0.0194).
Discussion
This is the first study to show that PWH with worse apathy and
other attributes of depressed mood had higher levels of GFAP in CSF.
Since in the central nervous system GFAP is found only in astrocytes,
and since its expression is upregulated in activated astrocytes [34],
higher CSF GFAP concentrations are believed to reflect greater
astrocyte activation. Astrocytes are known to be activated in HIV
infection [36-41] and to influence brain circuits involved in mood
and motivation [30,31,35]. This study’s principal finding that
depression in PWH was associated with higher CSF GFAP levels was
robust to consideration of a variety of important demographic and
disease-related potential confounds. Our data are consistent with
previous research on the role of astrocyte activation in depression
in PWoH [30,31] and extend these findings to PWH.Consistent
with the existing literature [44], worse depressed mood in this study
was associated with several adverse outcomes including poorer viral
suppression and independence in instrumental activities of daily
living, highlighting the clinical impact of depressed mood in PWH (Figure 1).
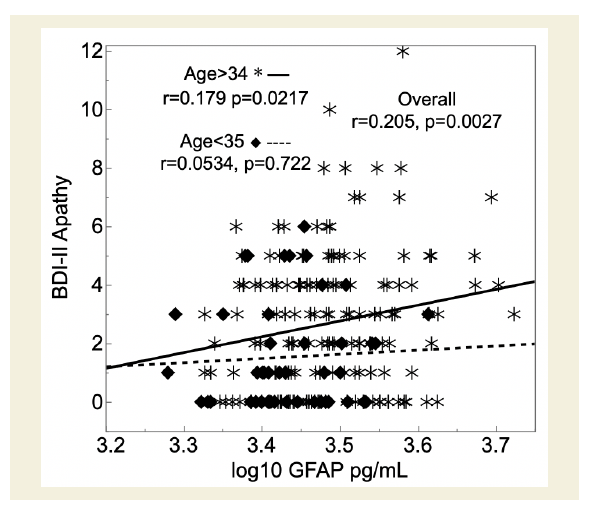
Figure 1: PWH with higher levels of glial fibrillary acidic protein (GFAP) in
cerebrospinal fluid (CSF; x-axis) had worse BDI-II apathy subscale scores
(y-axis). This relationship was significant for those older than 34 years
(asterisks), but not for younger participants (diamonds).
We suggest that the impact of astrocyte activation on depression
is via neurotoxicity [37]. Astrocytes, among other functions, are
responsible for metabolic support to neurons [45,46] and are involved
in neuronal repair [47]; activation of astrocytes diverts their resources
from neuronal support. Astrocyte activation related to HIV infection
may confer greater vulnerability to depression in PWH, a biological
risk factor that may explain the higher prevalence of depression in
PWH.
Strengths of this work include the careful characterization
of depressed mood and the consideration of a range of potential
confounding factors, to which the primary findings were robust.
The cohort was multicenter and racially diverse, enhancing
generalizability. Limitations of this study include its cross-sectional
design, limiting causal inference. Based on existing knowledge, a
causal link between astrocyte activation, as indexed by CSF GFAP,
and depressed mood is plausible; however, it is conceivable that
changes in activity, diet and other lifestyle factors associated with
depression might lead to astrocyte activation (reverse causation).
Statistical confounds were not detected in this study; however, an unmeasured variable might account for the association between
GFAP and depressed mood. The effect sizes demonstrated here were
small, albeit statistically significant. Females were underrepresented.
The rate of viral suppression was lower than in many modern cohorts;
however, after adjustment for viral suppression, elevated CSF GFAP
levels were still significantly associated with depressed mood.
Antidepressant medications could have been taken for reasons other
than depressed mood, such as for neuropathic pain.
These findings raise the possibility of interventions, potentially
influencing pathways that might affect depressed mood [48-51]. For
example, using a glucagon-like peptide-1 receptor (GLP-1R) agonist
[52,53] or the synthetic cannabinoid R(+)WIN 55,212-2, both of
which inhibit astrocyte activation [31,48]. A future clinical trial
may fruitfully explore this therapeutic option for depressed PWH,
particularly those who fail to respond to traditional antidepressant
treatments.
Acknowledgments
Supported by grants R01MH092673 (J. He), R01MH107345 (PIs
Heaton and Letendre), and R24MH129166 (PIs Letendre and Ellis)
from the National Institutes of Health, Bethesda, MD, United States.
The CNS HIV Anti-Retroviral Therapy Effects Research
(CHARTER) group is affiliated with Johns Hopkins University; the
Icahn School of Medicine at Mount Sinai; University of California,
San Diego; University of Texas, Galveston; University of Washington,
Seattle; Washington University, St. Louis; and is headquartered at
the University of California, San Diego and includes: Directors:
Robert K. Heaton, Ph.D., Scott L. Letendre, M.D.; Center Manager:
Donald Franklin, Jr.; Coordinating Center: Brookie Best, Pharm.D.,
Debra Cookson, M.P.H, Clint Cushman, Matthew Dawson, Ronald
J. Ellis, M.D., Ph.D., Christine Fennema Notestine, Ph.D., Sara
Gianella Weibel, M.D., Igor Grant, M.D., Thomas D. Marcotte, Ph.D.
Jennifer Marquie-Beck, M.P.H., Florin Vaida, Ph.D.; Johns Hopkins
University Site: Ned Sacktor, M.D. (P.I.), Vincent Rogalski; Icahn
School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (P.I.),
Letty Mintz, N.P.; University of California, San Diego Site: J. Allen
McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann
Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Sher
Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman,
M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington
University, St. Louis Site: David B Clifford, M.D. (P.I.), Mengesha
Teshome, M.D.The views expressed in this article are those of the
authors and do not reflect the official policy or position of the United
States Government.