Journal of Orthopedics & Rheumatology
Download PDF
Research Article
Cartilage Tissue Engineering: An Update on Multi-Component Approach
Zong Z1, Wu X1,2, Su Z1, Wang Z1, Zhao Z1, Huang J3, Zhong C4, Wei B1, Li G5 and Lin S1,2,6*
1Orthopaedic Center, Affiliated Hospital of Guangdong Medical
University, Guangdong Medical University, China
2Marine Biomedical Research Institute, Guangdong Medical University, China
3Department of Stomatology, Guangdong Medical University, China
4Institute of Laboratory Medicine, Guangdong Medical University, China
5Department of Orthopaedics and Traumatology, The Chinese University of Hong Kong, China
6Department of Orthopaedic Surgery, Stanford University, USA
2Marine Biomedical Research Institute, Guangdong Medical University, China
3Department of Stomatology, Guangdong Medical University, China
4Institute of Laboratory Medicine, Guangdong Medical University, China
5Department of Orthopaedics and Traumatology, The Chinese University of Hong Kong, China
6Department of Orthopaedic Surgery, Stanford University, USA
*Address for Correspondence: Lin S, Department of Orthopaedic Surgery, School of Medicine, Stanford
University, 300 Pasteur Drive, Edwards R163, Stanford, CA 94305, USA; E-mail: sienlin@stanford.edu
Submission: 08 November 2019;
Accepted: 05 December 2019;
Published: 12 December 2019
Copyright: © 2019 Zong Z, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Cartilage injury and osteoarthritis are big clinical challenges as
self-healing potential of cartilaginous tissue is very limited. The need
for a multi-disciplinary approach in order to establish new strategies
for cartilage healing has been addressed by many scientists from the
fields of orthopaedic surgery or biomedical engineering in the last two
decades. With a focus on the very preclinical research in this field,
this review covers the multitude of approaches, ranging from cell-based
to scaffold-based strategies and also including growth factors,
precondition approach, mechanical stimulation-that have been
combined to assess their potential to develop effective concepts for
the treatment of cartilage injury or osteoarthritis.
Keywords
Cartilage injury; Osteoarthritis; Regenerative medicine;
Stem cell-based therapy; Biomaterials; Growth factors
Epidemiology of Cartilage Injury and Osteoarthritis
Cartilage injury (also called chondral injury) is known as the
lesion within cartilage layer, while osteochondral injury is the fullthickness
lesion extending to the subchondral bone. Cartilage
injury or osteochondral injury is common in sport injuries [1], road
traffic accidents [2], and other trauma. An epidemiological study on
31,516 knee arthroscopies in USA reported that 63% of patients had
chondral lesions (averaging 2.7 lesions per knee) and 20% had fullthickness
lesions, with 5% of these occurring in patients less than 40
years of age [3], 65% of them were accompanied with meniscal or
ligament lesions, mostly anterior crucial ligament (ACL) tear [3,4]
In subgroup analysis, 75% of young patients below 40 years old
had solitary chondral lesions and; the remaining 25% had multiple
lesions. Another similar study conducted in Poland examining a total
of 25,124 knee arthroscopies, reported that chondral lesions were
found in 60% of these patients. Medial meniscus tear (37%) and ACL
injury (36%) were the most frequent associated factors [5].
Cartilage is categorized into three types including hyaline cartilage,
elastic cartilage, and fibro cartilage according to its composition.
Articular cartilage is a tough but flexible hyaline cartilage that covers
the ends of bones at a joint, which functions as a cushion allowing
smooth joint movement. As articular cartilage injuries can occur
focally, which is localized and contained, or globally, which can
finally lead to joint Osteoarthritis (OA) - the most common chronic
joint disease. OA is a chronic degenerative disease mainly happened
in elderly with destruction of articular cartilage and subchondral
bone sclerosis, which is distinct from acute cartilage injury. Data from
2010 to 2012 showed that one in five, or 52.5 million, USA adults
had arthritis; one in nine, or 22.7 million, had arthritis-attributable
activity limitations [6]. Recently, it was reported that more than fifty
million of the population over 60 years old in mainland China were
affected by joint pain that may be attributed to osteoarthritis [7]. A
local survey in Hong Kong on men aged 50 years and above revealed
that 17% and 7% had persistent knee pain and OA, respectively. The
prevalence in women was higher, being 24% and 13%, respectively
[8].
Healing Process of Cartilage Injury
Cartilage is an avascular tissue with minimal supply of nutrients
and progenitor cells from circulation, and composed of limited
number of chondrocytes with low mitotic potential, making cartilage
a poor self-regenerating tissue in response to injury [9]. In cartilage,
nutrients and wastes exchange are achieved through synovial
fluid perfusion, which also allow the delivery of various factors
participating in healing [9]. Scarce resident stem cells in cartilage
are identified recently, which require considerable manipulation
efforts to generate cartilage in vitro [10]. Chondroclasts have only
been described for calcified or hypertrophic matrices, which are
proposed to play a role in cartilage remodeling. Tiny defects are
healed by migration of chondrocytes, while large defects are healed by
formation of biomechanically incompetent fibrocartilage [11]. Hence,
cartilage lesions seldom heal spontaneously and thus constitute one
of the main causes of joint disease and disability [12,13]. Given that
persistent cartilage defects gradually lead to degeneration of the
articular cartilage and osteoarthritis [11], the restoration of cartilage
integrity through the promotion of cartilage regeneration has been a
research question over the decades.
Traditional Treatments for Cartilage Injury or Osteoarthritis
Primary treatments options including protecting from further
injury, ice cooling, and analgesic may help to settle the initial pain and
swelling after acute cartilage injury. Further surgical treatments are
subjected to the severity of cartilage lesion. Several surgical techniques
are readily available to treat cartilage injuries of the knee upon different scenarios [14]. Amount of all, operations like arthroscopic lavage,
debridement, microfracture, Autologous Chondrocyte Implantation
(ACI), and Osteochondral Autograft Transplantation (OAT) are
most widely used nowadays encountering to the cartilage lesions [15].
These reparative methods are tended to stimulate the formation of
new fibrocartilage tissue by facilitating access to the vascular system
and bringing new progenitor cells capable of chondrogenesis (e.g.,
microfracture procedure and drilling). Reconstructive methods fill
up the defects with autologous, homologous, or other tissue (e.g.,
autologous chondrocyte implantation and osteochondral autologous
transplantation) [16,17]. Such methods may associated with good
outcomes after surgery, but according to a systematic review of level I
and II studies on OAT procedures and microfracture surgery showing
that, patients with small lesions who returned to higher-demand
activities had an higher progressive failure rate and only 52% of
athletes returned to sports after received microfracture surgery, 37%
of them retained their same level of sports 10-year after operation
[18,19]. Besides, another systematic review reported by Filardo et al.
revealed that 33.7% failure rate at a mean was recorded follow-up of
8.5 years after ACI surgeries (5-12 years post-surgery) in 193 patients
[20].
The therapeutic strategies for OA are distinct from acute
cartilage injuries. Chronic pain relief could be achieved with
lifestyle modification and medication such as Non-Steroidal Anti-
Inflammatory Drugs (NSAIDs) or glucocorticoid. NSAIDs are the
most widely prescribed pharmacological medications and were
recommended in the guidelines in the treatment of OA but longterm
administration are associated with serious side effects including
bleeding and perforated gastric ulcers [21-23]. Long-term use of
glucocorticoid may cause several side effects such as immunodeficiency,
osteoporosis, peptic ulcer disease or gastrointestinal bleeding [24,25].
Viscosupplementation with hyaluronic acid through intra-articular
injection helps to reduce OA caused pain through its lubricating
action, but recent clinical studies showed that the use of hyaluronic
acid did not improve clinical outcomes compared to the placebo
group significantly [26,27].
However, these current treatments are not promising solution
to prevent articular cartilage from further progressive destruction,
thus OA patients may need joint replacement to regain reasonable
joint movement at the expense of potential complications. Although
the shelf life of prosthetics for joint replacement is significantly
improved, this surgery remains less suitable for young OA patients
[28,29]. Thus, there is a burning need for alternative approaches to
manage cartilage lesions, which would prevent the early onset of OA
and to reduce the need for total joint replacement.
Biological Solutions for Cartilage Repair
Autologous Chondrocyte Implantation (ACI) is a convincing
and effective method for the treatment of cartilage lesions [30,31].
The usefulness of allogeneic chondrocytes as alternative source
was constrained because of the reported immunogenicity [32].
Furthermore, in vitro expansion of chondrocytes can lead to rapid
dedifferentiation and a fibroblastic phenotype [30], resulting in an
inferior tissue-engineered cartilage.
Mesenchymal Stem Cells (MSCs) are a promising and readily available cell source showing chondrogenic differentiation potential
and forming cartilage-like tissues in vitro induced by specific growth
factors without compromising its low immunogenicity [33-37]. MSCs
can be derived from various types of tissues, including bone marrow
[38,39], adipose tissue [40], tendon [41,42], synovial membrane [43],
dental pulp [44], umbilical cord blood [45], placenta [46,47], etc.
Autologous MSCs are currently the major cell source because of
ethical and immunological concerns. However, a major drawback of
their clinical use is the aging-related decline in MSCs proliferation
and chondrogenic differentiation potential from aged patients
(donors) and in vitro cell culturing as several studies had reported
that MSC isolated from older donors exhibited a slower proliferation
rate throughout the entire in vitro expansion compared with the
younger donors. And the shorter average length of telomere, loss
of telomere length after cell passage and lower levels of telomerase
activity may contribute to such phenomenon. Besides, the expression
of p16INK4A is also strongly associated with cell senescence [48-51]. Furthermore, instable MSCs phenotypes such as formation of
mineralized deposits within cartilage. Current available strategies
for enhancing plasticity of MSCs included genetic modification [52-54], hypoxia stimulation [55,56], etc. However, safety and ethical
concerns are existed for genetic modification approach, which is
left far behind clinical use, and hypoxia could only promote cell
proliferation at this stage. Hence, it is mandatory to find out a simple
and feasible manipulation for promoting plasticity of MSCs including
proliferation, chondrogenesis and viability.
Dedifferentiation Reprogrammed MSCs for Tissue Regeneration
Cellular dedifferentiation is cellular regression from a more
differentiated stage back to a less differentiated stage from within its
own lineage that confers pluripotency, giving rise to reminiscent of
stem cells [57,58]. Based on this definition, cellular dedifferentiation
is not only initiating from a completely differentiated stage, but
also initiating from partially differentiated stage. Similarly, cellular
dedifferentiation could result in partially or fully pluripotent
cells, depends on the different time points. This process is more
commonly studied in plants and more primitive creatures. Several
non-mammalian vertebrate species, such as zebra fish and urodele
amphibians [59-65], possess a remarkable capacity to regenerate heart
tissue or limb, respectively. Apart from natural conditions, researchers
found that inducible dedifferentiation is an appropriate strategy to
promote regeneration in mammalian tissues that lack of this ability.
Studies have reported the occurrence of cell dedifferentiation during
tissue regeneration both in vitro and in vivo [66-70].
Recent studies have demonstrated that dedifferentiation
reprogramming is a reliable method to improve properties of stem
cells and promote lineage differentiation commitment [71-73].
Previous data revealed that a population of MSCs with enhanced
viability in vitro and improved therapeutic efficacy in a cerebral
ischemia model could be attained via neuronal differentiation and
dedifferentiation reprogramming [72]. Recently we reported that,
compared with untreated MSCs, MSCs which manipulated with
osteogenic differentiation medium exhibited a better osteogenic
differentiation potential, improved cell migratory capacity and upregulated
expression of genes Nanog, Oct4 and Sox2 [74].
And we also proved that such improvements were inducted by decreased
methylation and accrual of activating histone marks of promoters
on Nanog and Oct4.Besides, after preconditioned with chondrogenic
differentiation medium and complete medium, the Manipulated MSCs
(M-MSCs) also showed an improved cell clonogenicity, proliferation,
survivability and chondrogenic property. And the results of
epigenetic analysis revealed the central role of Nanog in maintaining
the multipotency of the manipulated MSCs [75]. Furthermore, we
also revealed that neocartilage formation of M-MSC-laden constructs
implanted in the nude mice was significantly promoted after dynamic
compressive applied in the bioreactor and the constructs laden with
M-MSCs were also significantly promoted the cartilage healing
process of osteochondral defect of a rat model [76].
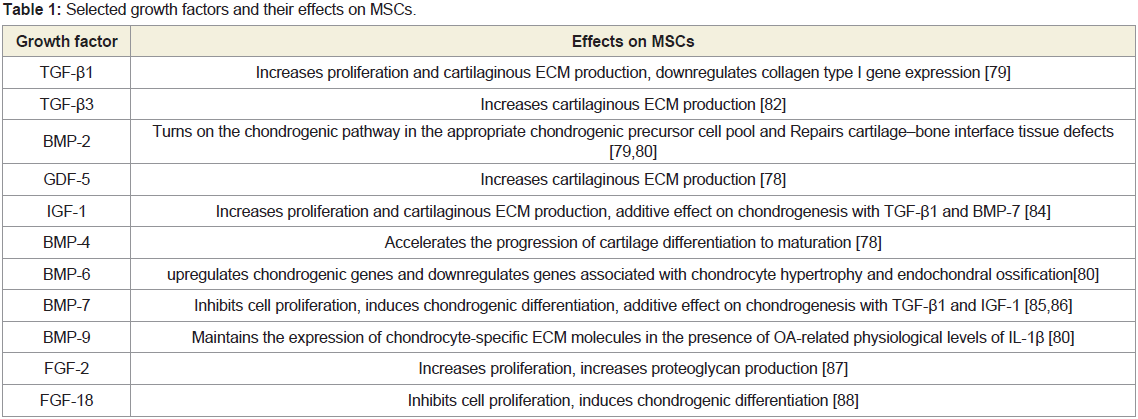
Growth Factors for Chondrogenic Differentiation
In the hyaline cartilage, growth factors regulate homeostasis
and integrity, as well as development [77]. Growth factors also play
an important role in the process of chondrogenic differentiation
of MSCs. (Table 1) summarizes some representable endogenous
bioactive cytokines, including Transforming Growth Factor β
(TGF-β) superfamily with respect to cartilage tissue engineering are
TGF-β1, TGF-β3, Bone Morphogenetic Protein 2(BMP-2), BMP-4,
BMP-6, BMP-7, BMP-9 and Growth Differentiation factor-5 (GDF-
5) [78-81], which are reported to stimulate MSCs proliferation
and differentiation. Among of these, TGF-β1 and TGF-β3 are the
most frequently used cytokines in experimental studies to promote
chondrogenic differentiation and synthesis of corresponding
Extracellular Matrix (ECM) production [79,81-88] (Table 1).
Biomaterials for Cartilage Repair
Various materials in the form of sponges, hydrogels, electrospun
fibers, and microparticles have been fabricated as scaffolds to support
chondrogenic differentiation [89]. Natural biomaterials derived
from either polymer (agarose, alginate, chitosan, and hyaluronan) or
protein (collagen, gelatin, fibrin, and silk) are biocompatible but have
poor mechanical strength and relatively high degradation rate in most
cases without proper modification [90,91]. Synthetic biodegradable
polymers offer some important advantages such as controllable
degradation rate, high reproducibility, high mechanical strength, and easy manipulation into specific shapes. However, the cell recognition
signals are usually missing in such scaffolds [92]. When stem cells
are applied to cartilage defects, direct administrations of stem cells
into cartilage defects often lead to limited cartilage regeneration due
to significant cell loss and death as a result of the harsh mechanical
loading and catabolic factors in the diseased joints [93]. The lack
of a functional carrier material to provide physical retention and
biochemical cues to the delivered cells in the cartilage defects results
in poor retention, significant death and unsatisfactory differentiation
of the cells [94]. Therefore, there exists a huge demand for effective
carrier biomaterials that afford not only physical support but also
biochemical signals to the delivered cells in order to promote the
cartilage repair. As articular cartilage is totally covered by the articular
capsule, it will be much helpful to deliver the cells through a minimal
invasive way, such as intra-articular injection.
Among all of these materials, natural polymer like Hyaluronic
Acid (HA) has been intensively investigated. HA can be modified
to photo-crosslink into 3D hydrogels that confers chondrogenesis
properties of MSCs [95]. The superior mechanical stiffness and
network porosity and permeability have positive impact on the
differentiation of encapsulated MSCs [96-99], distribution of newly
synthesized cartilage matrix, and nutrition transportation [100,101].
Previous data showed enhanced chondrogenic differentiation and
inhibited hypertrophy could be achieved by modulating cross linking
density of HA macromer [102,103]. Besides, after modified Quantum
Dots (QDs) with β-Cyclodextrin (β-CD) and RGD peptide, the
manipulated nanocarrier gained the ability of carrying hydrophobic
small molecules such as kartogenin in the hydrophobic pockets
to induce chondrogenic differentiation of human mesenchymal
stem cells [104]. Moreover, after conjugated sulfate groups to HA,
these modified sulfated HA exhibit a higher protein affinity and
significantly slower degradation by hyaluronidase with no negative
effect on the viability of human Mesenchymal Stem Cells (hMSCs)
compared to the wild type HA hydrogel, which results the avert of
cartilage abrasion and hypertrophy in the osteoarthritis joints of a rat
model of OA [105].
Compared with HA, after proper modification, gelatin hydrogel
also exhibited an excellent capacity of self-healing and improved physical and biological properties. Recently, cyclodextrin-based
host-guest interact with gelatin are of great interest because of its
effectiveness and specificity of host-gust molecular recognition
under physiological condition which can be facilitated to form
supramolecular hydrogels. In our recent study, we revealed that
thought crosslinked acrylated β-cyclodextrins (Ac-β-CDs) with the
aromatic residues of gelatin by in situ formed multivalent host-guest
nanoclusters under UV-initiated oligomerization, the as-prepared
hydrogel shown a significantly enhanced mechanical strength
thanks to its reversible nature of the host-guest interactions. Those
interactions enables the hosts and guests moieties to re-form the hostguest
cross-links thus preventing the early rupture of the polymer.
Besides, the Host-Guest Macromer (HGM) hydrogels also exhibited
improved compressive properties with much faster stress relaxation
rate. Such enhanced compressibility and fast stress relaxation property
facilitate the HGM hydrogels to fit into irregular geometries without
compromising the hydrogel integrity [106]. Moreover, the Host-
Guest Macromer (HGM) hydrogels were also able to sustain release
of encapsulated therapeutic growth factors and deliver therapeutic
cells. In animal study, we also demonstrated that such novel HGM
hydrogel could significantly promoted the cartilage regeneration in
a rat model [106]. In our subsequent study, we also demonstrated
that the injectable stem cell-laden HGM hydrogels could remarkably
boost the regeneration of both cartilage and subchondral bone in an
osteochondral defect model after encapsulated human Bone Marrowderived
Mesenchymal Stem Cells (hBMSCs) with small molecule
(Kartogenin) and proteinaceous chondrogenic agents (TGF-β1).
Data also showed that the injection process only has a minor negative
impact on cell viability and chondrogenic differentiation capacity
of the cells encapsulated in the hydrogels which indicated that such
biomaterial and cell delivery method could greatly facilitate stem cell
therapies [107].
Mechanical Stimulation and Chondrogenic Differentiation
Mechanical stimulation with bioreactors on cell-seeded constructs
is a well-established cue for improving the mechanical properties of
tissue-engineered cartilage [108,109]. Direct confined or unconfined
compression and hydrostatic pressure are the two most investigated
loading regimes in cartilage tissue engineering studies. Direct dynamic
compression applied to chondrocyte-seeded constructs generally
increased ECM production and proliferation of chondrocytes, and
improved compressive properties of the engineered tissue [110-117].
Mechanical forces generated intrinsically within the cell in response
to its extracellular environment, and extrinsic mechanical signals
imposed upon the cell by the extracellular environment, play a critical
role in determining the fate of MSCs [118-120]. Mechanical signals
have also been reported to induce chondrogenesis of bone marrow derived
MSCs and inhibit subsequent hypertrophy as effectively as
TGF-β1 stimulation [121-125]. Compressive loading is the most
frequently used protocol for promoting chondrogenesis of MSCs.
A combination of TGF-β1 and compressive loading presents a
synergistic effect on chondrogenic differentiation [126]. Apart from
compressive loading, fluid flow has also been shown to upregulate
Sox9 gene expression in murine C3H10T1/2 MSCs plated onto glass
slides [127]; tensile strain regulated chondrogenic differentiation and
GAG synthesis by MSCs embedded in collagen-GAG [128].
Conclusion
With aging and rising of obesity, cartilage injury and
osteoarthritis has become major healthcare problem worldwide.
The biological approaches showed a great therapeutic potential in
the treatment of cartilage injury or OA. However, open questions
and challenges are existed and remained to be settled, as most of the
studies are still at early stage and evidences such as long-term and
large-scale study are still needed. Besides, the problem of stability
of the growth factors, survival rate of the cells encapsulated in the
biomaterial and large-scale fabrication are still challenging the
process of final commercialization. Taken all these together, till now,
even bioactive scaffold cannot completely meet every request in the
clinical application; we still believe that biological functionalization
solutions are the future direction for the treatment of cartilage injury
and osteoarthritis.