Journal of Parkinsons disease and Alzheimers disease
Download PDF
Research Article
Effects of Physical Exerciseon Cognition in Persons with Subjective Cognitive Decline or Mild Cognitive Impairment: A Review
Sangwoo Ahn*, Dereck Salisbury and Fang Yu
- School of Nursing, University of Minnesota, USA
*Address for Correspondence: Sangwoo Ahn, School of Nursing, University of Minnesota, 925 Delaware St SE, Minneapolis, MN 55414, USA, Tel: 1-651-605-1485; Fax: 1-612-625-7180, E-mail:ahnxx230@umn.edu
Citation: Ahn S, Salisbury D, Yu F. Effects of Physical Exercise on Cognition in Persons with Subjective Cognitive Decline or Mild Cognitive Impairment: A Review. J Parkinsons Dis Alzheimer Dis. 2017;4(2): 11.
Journal of Parkinson’s disease & Alzheimer’s disease| ISSN: 2376-922X | Volume: 4, Issue: 2
Submission: 14 September, 2017| Accepted: 6 October, 2017, | Published:16 October, 2017
Submission: 14 September, 2017| Accepted: 6 October, 2017, | Published:16 October, 2017
Abstract
Alzheimer’s disease is the most common type of dementia that lacks a cure so prevention is an important aspect of care. Persons with subjective cognitive decline or mild cognitive impairment are more likely to be diagnosed with Alzheimer’s disease later than cognitively healthy populations. The purpose of this literature review was to analyze the effects of physical exercise interventions on cognition in persons with subjective cognitive decline or mild cognitive impairment. A literature search was conducted using electronic databases from the beginning dates of the databases to September-23-2016. Of the 94 randomized controlled trials generated 6 subjective cognitive decline and 9 mild cognitive impairment studies met the eligibility criteria and were included in this review. The levels of study quality for subjective cognitive decline and mild cognitive impairment were 5 strong 1 moderate and 5 strong 4 moderate respectively. Interventions were aerobic, resistance, Tai Chi or multicomponent exercise. Overall 67%(10/15) of the studies showed that the interventions improved cognition compared to control or other interventions. There is a pressing need to conduct further research to establish the optimal dose of each modality for improving cognition in these populations.
Keywords
Subjective cognitive decline; Mild cognitive impairment; Exercise; Cognition
Introduction
The rising prevalence of dementia is a global health issue. Worldwide about 47.5 million have dementia in 2016 and almost 7.7 million new cases are expected to occur every year [1]. The totalannual costs associated with dementia are $226 billion in 2015 and estimated to exceed $1 trillion US dollars in 2050 in the United States [2]. Among all kinds of dementia, Alzheimer’s Disease (AD) is the most common type and accounts for 60-80% of all dementias [1].
The National Institute on Aging-Alzheimer’s Association (NIAAA) introduced a model of the clinical trajectory of AD, including preclinical AD, Mild Cognitive Impairment (MCI) due to AD and AD [3,4]. Preclinical AD refers to the stage at which AD pathology is present, but results from standardized cognitive tests represent normal performance [4]. Self-perceived decline in any cognitive domain may occur in this stage called Subjective Cognitive Decline (SCD) in preclinical AD [4,5]. Even if SCD can be a reflection of subtle cognitive decline in preclinical AD cognitive decline does not reach the threshold level of MCI based on the cognitive tests [5]. However the risk of developing AD or other dementias is higher in those with SCD compared with those without SCD [6-8].
MCI is diagnosed when objective decline in cognition exists but does not meet the criteria for a diagnosis of dementia [9,10]. Core clinical characteristics include: a) Self or informant reported cognitive complaints b) Objective cognitive impairments c) Preserved general cognitive functioning d) No evidence of dementia [11]. MCI due to AD represents cognitively symptomatic but non-demented state whose primary underlying pathophysiology is AD [3]. The presence of MCI puts individuals at a higher risk of developing AD with annual estimates ranging from 10% to15% compared to 1% to 2% among cognitively normal individuals [12].
There is no specific cure for AD. Medications such as acetylcholinesterase inhibitors and N-methyl-d-aspartate antagonist are prescribed for persons with AD to improve cognition, but they are not disease modifying treatments [13]. Therefore delaying or slowing down the onset of AD would be important to reduce burdens to the society and patients inflicted with this illness. However, there are no current established preventive medications for AD [13,14].
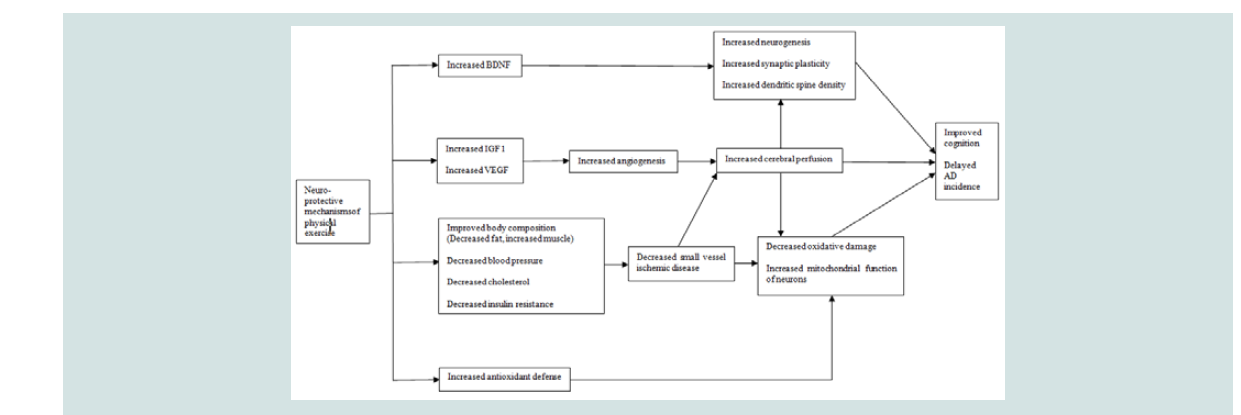
In this circumstance non-pharmacological interventions have been tested to improve cognition in persons before the onset of AD. Physical exercise is one of these non-pharmacological interventions and its impact on cognition is known to be beneficial in non-demented persons [15-17]. There are biologically sound mechanisms that support the beneficial effects of physical exercise on cognition [18]. Specifically physical exercise has been shown to up-regulate several factors linked to neuro-protective mechanisms including Brain- Derived Neurotrophic Growth Factor (BDNF) Insulin-Like Growth Factor (IGF) Vascular Endothelial Growth Factor (VEGF) body composition and antioxidant defense. These changes are followed by decreased oxidative damage as well as increased neurogenesis, synaptic plasticity, dendritic spine density, cerebral perfusion and mitochondrial function of neurons. With benign effects derived from these mechanisms physical exercise can eventually have potential to improve cognition (Figure 1). Thus physical exercise can be an efficient option to diminish the risk of cognitive decline delaying the resence of AD.
Figure 1:Theoretical model of mechanisms linkage between physical exercise and cognition. AD=Alzheimer’s Disease; BDNF=Brain-Derived Neurotrophic growth factor; IGF=Insulin-Like Growth Factor; VEGF=Vascular Endothelial Growth Factor.
Purpose
Hence research targeting persons in stages potentially before AD particularly in SCD or MCI may be recommended to slow down AD progression. However the concepts of SCD and MCI have been defined in a variety of ways from study-specific clinical criteria to recommendations of the international workgroups [5,11,19]. There have been reviews which focused on aerobic exercise effects on cognition in MCI [19,20]. In order to fill the gap with updated evidence the purpose of this review was to describe what is known about the cognitive impact of various types of physical exercise specifically on persons with SCD or MCI diagnosed with strict criteria.
Methods
Ovid MEDLINE and Ovid EMBASE databases were searched from the inception of the database through September-23-2016 by combining keywords matched to Medical Subject Headings (MeSH):“Subjective cognitive complain or subjective cognitive decline or subjective cognitive impairment or subjective memory complaint or subjective memory impairment or subjective memory decline or self-reported memory failure or mild cognitive impairment” and “exercise or exercise movement techniques or exercise therapy or sports oraerobic activity/fitness/training or resistance exercise/ training or strength exercise/training or walkingor running or jogging or swimming or Pilates or yoga or Tai Chi or dance or breathing exercise or stretching or balance training or postural training” and “cognition or cognitive function”. Searches were limited to human studies and published articles in all databases.
Articles were included if: 1) studies were published in English 2) studies used a Randomized Controlled Trial (RCT) design 3) participants had SCD or MCI 4) studies dealt with physical exercise interventions 5) Cognition or cognitive function was measured as an outcome.
The exclusion criteria were: 1) Studies published in a language other than English 2) Review types of commentary, non-intervention study, quasi-experimental design or case study 3) Participants without SCD or MCI 4) Interventions that did not contain physical exercise 5) Outcomes other than cognition or cognitive function.
This review defined SCD using the criteria from the Subjective Cognitive Decline Initiative (SCD-I) [5]. Persons with SCD should have: 1) Self-experienced persistent decline in cognitive capacity in comparison with a previously normal status and unrelated to an acute event 2) Normal age, gender and education-adjusted performance on standardized cognitive tests, which are used to classify MCI or prodromal AD 3) No MCI, prodromal AD and dementia and 4) SCD that cannot be explained by a psychiatric condition, neurologic disease, medical disorder, medication, or substance use. According Vega et al. there are 3 criteria which are currently recognized to diagnose MCI [11]. This review defined MCI using either of these 3 criteria from: 1) the Mayo Clinic [9,10] 2) the NIA-AA on MCI due to AD [3] or 3) the fifth edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) [21].
Global cognition or at least one domain of specific cognitive abilities (eg. executive function, learning, memory, attention and language) was assessed by standardized neurocognitive tests or other objective measures (Table 1 andTable 2).The matrix system after Garrard was used to summarize the included studies [22]. Lead author’s name, date, purpose, study design, sample size, setting, outcomes/measures, and results were extracted (Table 1 andTable 2). The quality of studies was assessed using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies [23]. Ratings included: strong quality (No weak ratings among 6 categories) moderate quality (One weak rating) or weak quality (two or moreweak ratings). The EPHPP has 6 categories including selection bias, study design, confounders, blinding, data collection method and withdrawals. Each category is graded as ‘strong’, ‘moderate’ or ‘weak’. he category ratings together gave an overall quality rating (Table 1 andTable 2).
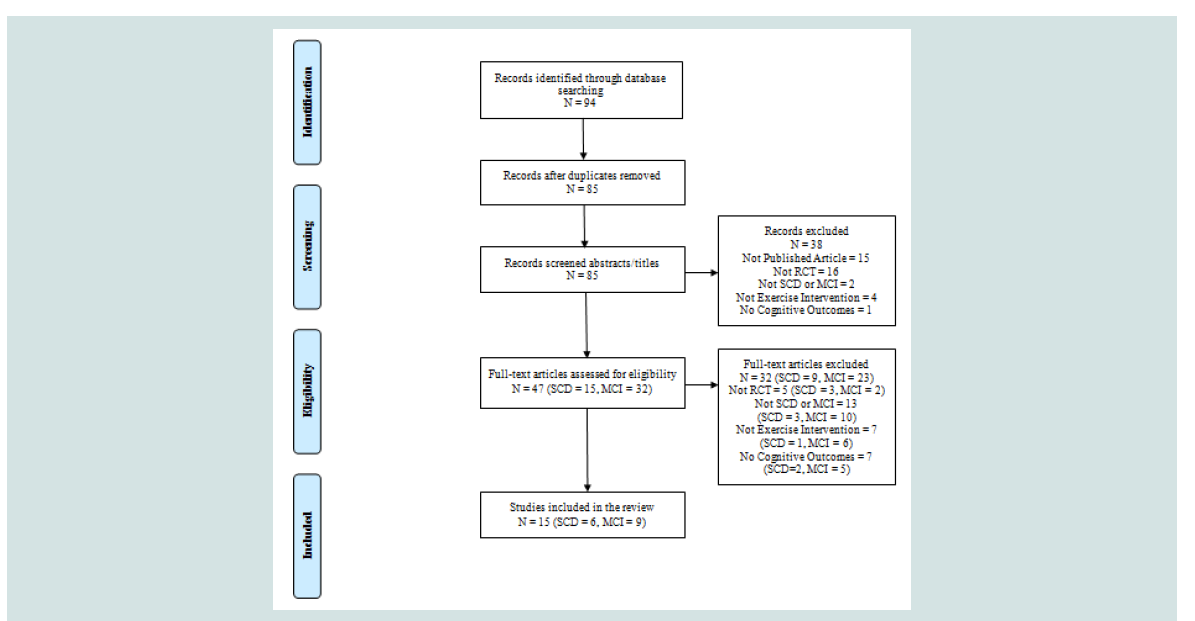
A total of 94 articles were generated and 9 duplicates were removed. Of those 85 articles 47 remained after abstracts were screened for eligibility using the inclusion and exclusion criteria by the reviewer (SA). The reviewer read those 47 full text articles to assess the eligibility. inally a total of 15 (SCD=6, MCI=9) met the eligibility criteria and were included in this review (Figure 2).
Results
The effects of physical exercise on cognition in SCD
A total of 6 SCD studies were included in this review. Two of 6 studies were conducted in Canada (n=2) with the remaining 4 conducted in Australia (n=1) China (n=1) Japan (n=1) and the United States (n=1) [24-29]. Five of the 6 studies were rated with strong quality and 1 with moderate quality (Table 1) . The mean ages in the 6 studies ranged from 69 to 75. Among the 6 studies 2 used aerobic exercise, 1 used resistance exercise, 2 used either aerobic or resistance exercise and 1 used multicomponent exercise (i.e., combined aerobic/ resistance exercise) [24-29]. Five of the 6 studies used supervised exercise but 1 study did not [26]. The exercise dropout rates were 5%, 12%, 19% or 7% to 20%. The exercise adherence rates were 78%, 80%, 86%, 88% or 54% to 60% [24-29]. The reasons for dropout included illness and unwillingness to continue [24-26,29]. While factors affecting exercise adherence were not reported in all included studies 1 study provided a social cognitive theory-based intervention package which comprised information on exercise programs, rewards, goal setting, time management, barriers to activity and safe exercise in order to enhance adherence [26]. No adverse events directly related to interventions were reported in the included SCD studies.
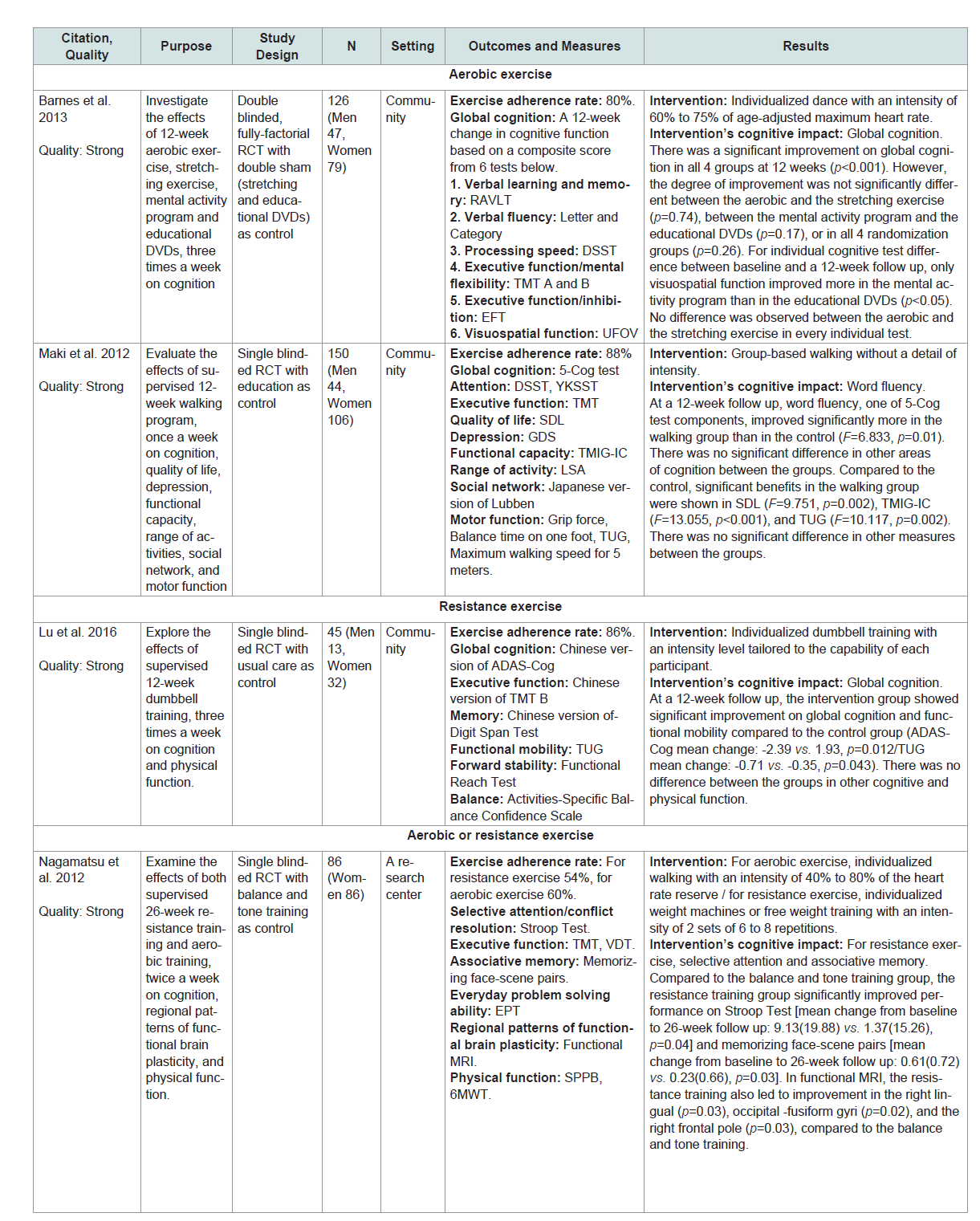
Five of the 6 studies (83%) in SCD showed that the physical exercise intervention used significantly improved cognition compared to control or other interventions[24-28]. For aerobic exercise each dose had its own effect on cognition. For example a 3-month group-based walking without a detail of intensity (90 minutes per session, once a week) significantly improved word fluency compared to an education [28]. A 6-month individualized walking with an intensity of 40% to 80% of the heart rate reserve (60 minutes per session, 2 times a week) was efficacious for improving verbal and spatial memory compared to a balance and tone training [24]. However a 3-month individualized dance with an intensity of 60% to75% of age-adjusted maximum heart rate (60 minutes per session, 3 times a week) resulted in global cognition improvement that was equally beneficial with a stretching [29].
For resistance exercise each dose also had its own effect on cognition. For example a 6-month individualized weight machines or free weight training with an intensity of 2 sets of 6 to 8 repetitions (60 minutes per session, 2 times a week) significantly improved selective attention, associative and spatial memory compared with a balance and tone training [24,25]. 3-month individualized dumbbell training with an intensity level tailored to the capability of each participant (60 minutes per session, 3 times a week) significantly improved global cognition compared to a regular lifestyle routine [27]. For multicomponent exercise, global cognition and episodic memory were significantly improved in a 6-month individualized walking and strength training without information on intensity (50 minutes per session, 3 times a week) compared with an education [26].
Based on the results of introduced interventions aerobic exercise was effective in improving word fluency and verbal memory while resistance exercise improved selective attention and associative memory. Both aerobic and resistance exercise improved global cognition and spatial memory. Multicomponent exercise (i.e., combined aerobic/resistance exercise) was effective in improving global cognition and episodic memory.
The effects of physical exercise on cognition in MCI
A total of 9 MCI studies were included in this review. Three of 9 studies were conducted in China (n=3) with the remaining 6 conducted in Australia (n=1) Japan (n=2) the Netherlands (n=1) and the United States (n=2) [30-38]. Five of the 9 studies were rated with strong quality and 4 with moderate quality (Table 2). The mean ages in the 9 studies ranged from 65 to 78. All 9 studies used the Mayo Clinic criteria for MCI diagnosis. Among the 9 studies 3 used aerobic exercise, 1 used resistance exercise, 2 used 24-form simplified Tai Chi a form of mind-body exercise and 3 used multicomponent exercise (i.e., combined stretching/aerobic/mind-body exercise or combined resistance/aerobic/balance exercise [30-37]). Seven of the 9 studies used supervised exercise. The remaining 2 Tai Chi studies used supervision for 8 to 12 or 4 to 6 weeks but did not use supervision after this period [31,32]. The exercise dropout rates were 4%, 6%, 10%, 17%, 21%, 22%, 27%, 35% and 46% [30-38]. The exercise adherence rates varied from 63%,75%, 79%, 81%, 82%, 86% to 94% with 2 studies without information of adherence rate [30-37]. The reasons for dropout included illness and unwillingness to continue while 1 study reported that the adherence rate was higher in persons living with a partner [36-38]]. No adverse events directly related to interventions were reported in the included MCI studies.
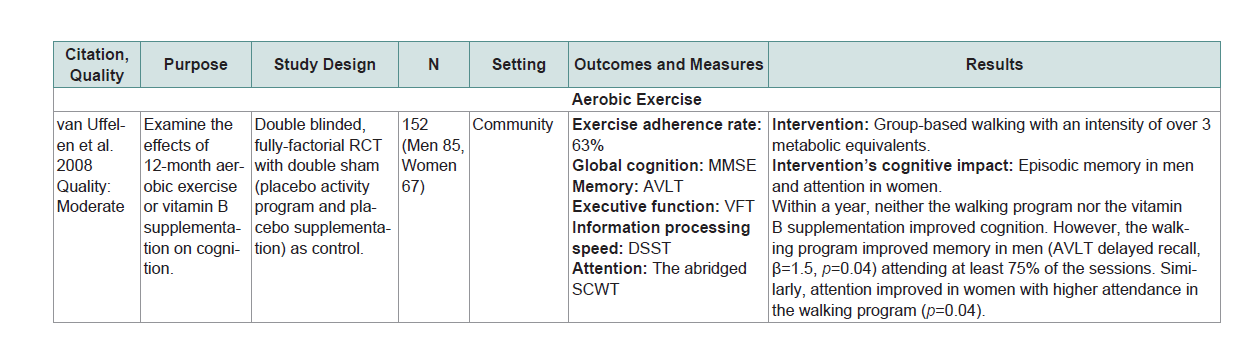
Five of the 9 studies (56%) in MCI showed that the physical exercise intervention used significantly improved cognition compared to control or other interventions [31-34,38]]. For aerobic exercise each dose had its own effect on cognition. For example a 6-month individualized treadmill walking with anintensity of 50% to 85% of the maximum heart rate (60 minutes per session, 3 times a week) significantly improved global cognition compared with a regular lifestyle routine[38]. A 6-month individualized treadmill walking or stationary bicycle riding with an intensity of 75% to 85% of the heart rate reserve (45 to 60 minutes per session, 4 times a week) was efficacious for improving executive function compared to a stretching but the effect was more prominent in women than men [38]. In addition a 12-month group-based walking with an intensity of over 3 metabolic equivalents (60 minutes per session, 2 times a week) resulted in episodic memory improvement in men and attention improvement in women in those with better adherence [36].
For resistance exercise a 6-month individualized weight machines or free weight training with an intensity of 15 to 18 on the Borg Scale (75 minutes per session, 2 times a week) significantly improved global cognition, executive function and visual/constructional memory compared with a combined cognitive/resistance or a cognitive training [33]. For Tai Chi effects on cognition varied by a durarion of interventions. A 5-month group-based 24-form simplified Tai Chi without a detail of intensity (at least 30 minutes per session at least 3 times a week) was efficacious for improving attention and global cognition compared to a stretching whilethe same dose of a 12-month program significantly improvedglobal cognitioncompared with a stretching [31,32].
For multicomponent exercise a 12-month group-based combined stretching/static bicycle riding/Tai Chi exercise without a detail of intensity (60 minutes per session, 3 times a week) significantly improved global cognition, episodic memory and language however a combined physical/cognitive activity showed better improvement on language than a multicomponent exercise [30]. Meanwhile cognitive gains differed by a duration of interventions. For example among participants with MCI a 6-month group-basedcombined muscle strength/walking/balance exercise with an intensity of average 60% of the maximum heart rate (90 minutes per session, 2 times a week) significantly improved global cognition and immediate memory compared to a health education in participants with amnestic MCI only where as the same dose of a 12-month program for participants with amnestic MCI significantly improved global cognition, immediate memory and language compared with a health education [34,35].
Based on the results of introduced interventions aerobic exercise was effective in improving executive function, episodic memory and attention but gender or adherence rate can play a role in an efficacy of this mode. Resistance exercise improved executive function and visual/constructional memory. Tai Chi was effective in improving attention. Aerobic, resistance and Tai Chi exercise all improved global cognition. Cognitive gainsof multicomponent exercises were similar (i.e., global cognition, episodic or immediate memory and language) despite different components: combined stretching/aerobic/mindbody exercise versus combined resistance/aerobic/balance exercise.
Discussion
This review examined the effects of physical exercise interventions on cognition in persons with SCD or MCI. Introduced interventions were aerobic, resistance and multicomponent exercise (i.e., combined aerobic/resistance exercise) for SCD as well as aerobic, resistance, Tai Chi and multicomponent exercise (i.e., combined stretching/aerobic/ mind-body or combined resistance/aerobic/balance exercise) for MCI. In these populations the findings of this review indicate that physical exercise interventions can improve cognition. Overall 67% of the studies (5 of the 6 studies in SCD and 5 of the 9 studies in MCI) indicated that the physical exercise intervention used significantly improved cognition compared to control or other interventions. Although the characteristics of samples were heterogeneous among studies, different doses of aerobic or resistance exercise had different cognitive outcomes in persons with SCD. However this finding cannot be overestimated as the dose-response relationships of aerobic or resistance exercise for SCD. An equivalent benefit on global cognition occurred between aerobic exercise and the control in 1 SCD study [29]. Difference between the groups may emerge in a different dose. A multicomponent exercise consisting of combined aerobic/resistance exercise improved global cognition and episodic memory [26]. It is possible that aerobic and/or resistance exercise may improve global cognition and/or episodic memory in the dose of this multicomponent exercise. It is noteworthy that the samples of 2 studies for SCD were only comprised of women [24,25]. Therefore an interpretation for the influence of either aerobic or resistance exercise in SCD can be restricted.
One multi component exercise and 1 aerobic exercise lack information on specific intensity [26,28]. This may make it hard to replicate interventions in the future. It is significant to see whether interventions’effects on cognition are prolonged as well as to help participants comply with exercise programs continuously. However most of the studies did not measure outcome variables beyond the end of the intervention period and only 1 study adopted a method (i.e., a theory-based approach) to facilitate exercise resulting in 78% of adherence rate [24,25,27-29]. A health behavior theoryguided approach is known to be more effective for facilitating health behavior (e.g., exercise) than a-theoretical way [39]. In this review this approach may help increase participants’adherence rate given that 2 studies without any theoretical basis showed relatively low adherence rate (i.e., 54% to 60%) [24,25].
For MCI the current findings of improved global cognition and executive function from aerobic exercise interventions are consistent with previous reviews but this review added new evidence of the effects of modalities other than aerobic exercise [19,20]. Similar to aerobic exercise in SCD the effects of aerobic exercise on cognition in persons with MCI varied depending on each dose although it should be interpreted with caution due to hetero geneous sample characteristics among studies. A gender-specific intervention effect was shown in 1 aerobic exercise study indicating a better effect on executive function in women [37]. This sex difference in cognitive response may be derived from metabolic effects of exercise. It was indicated that aerobic exercise enhanced gluco regulation and insulin sensitivity for women but not for men [37]. However this result cannot be generalized by 1 study with a small sample size (n=33).
Tai Chi is considered to be a light to moderate intensity exercise with estimated metabolic equivalents between 1.5 and 4.0 [40]. It makes comparison of the effects across exercise modes available although no specific Tai Chi intensity was reported in the included studies. The effects of introduced multicomponent exercises on cognition (i.e., improved global cognition, episodic or immediate memory and language) might come from a single mode of intervention (i.e., aerobic, resistance or Tai Chi exercise) in the specific dose of each multicomponent exercise. However it is also possible that cognitive gains could be earned by stretching or balance exercise.
It is important to note that the samples of 4 studies were only comprised of persons with amnestic MCI [31,32,34,37]. Therefore the effects of Tai Chi aerobic and multicomponent exercise (i.e., combined resistance/aerobic/balance) on cognition cannot be applicable to non-amnestic MCI. A majority of studies in MCI did not measure cognition after the intervention period [30-32,34,37,38]. A longer follow up time may permit identification of consistent effects of intervention on cognition. Given that persons living with a partner reported higher adherence rate monitoring from people in a trustful relationship may help participants adhere to exercise [36]. In addition considering the fact that one of the reported reasons for dropout was unwillingness finding special ways such as regular reminders or motivation to target persons with MCI may be recommended because cognitive gains can be greater in participants who conform with the exercise program [36,38]. Given that persons with MCI preserve their independence of function in daily life and more than 76% of older adults aged 65 and over own cell phones in the United States, a motivational mobile text message which was effective in increasing walking count among non-demented older adults can be an useful option [3,41,42].
Strengths and limitations
This review has comprehensively examined various modes of physical exercise and their effects on cognition in persons with SCD or MCI. However this review has some limitations. First the SCD studies in this review might include MCI populations. They completely excluded dementia but did not treat MCI criteria except for 1 study which included some MCI populations [26]. Second some cognitive measures assessing the same cognitive domain differ from study to study so the results were not always directly comparable to each other even if tests were designed to measure the same cognitive domain. Third this review is limited by the scope of the literature search. It is possible that some relevant literatures are published in databases other than Ovid MEDLINE and Ovid EMBASE. Finally the literature search was only concentrated on studies published in English. Therefore certain relevant articles might be omitted.
Implications for research and practice
This review has several implications for research. To begin with more samples defined using criteria from the NIA-AA and SCD-I are required to see the effects of physical exercise on cognition in the clinical trajectory of AD [3,5]. Future studies can adopt ADassociated biomarkers to include these samples. AD-associated biomarkers for example low Cerebrospinal Fluid (CSF) Beta-amyloid protein (Aβ) level and hippocampal volume atrophy can be found in persons with SCD or MCI indicating a high likelihood of progression to AD [3,43]. In addition, given that this review did not deal with a biological basis of how to improve cognition presented in Figure 1, incorporating biological variables is recommended to see which modality can explain a certain mechanism in these populations.
Studies for SCD or MCI also need to identify the effectiveness of interventions by gender or MCI sub-types. For intervention dose future research should not omit dose information because the effectiveness of interventions can be readily translated to practice by reporting exact exercise dose. Future studies should also reflect on this review to further test the dose-response relationship as well asthe minimally-effective or optimal dose of each modality in order to improve cognition for persons with SCD or MCI. For measures and follow up periods more research is needed to measure each cognitive domain using the same standardized tools to directly compare between studies. A longer follow up period beyond the end point of the intervention is also needed because a maintenace of positive effects on cognition could give a hint for better modality or dose.
This review has clinical implications. Although there is no consensus regarding an optimal exercise mode or dose, clinicians can refer to the findings of this review when prescribing physical exercise to persons with SCD or MCI to improve cognition. After discussing the potential benefits and risks clinicians can choose appropriate modality and dose based on each person’s age, medical frailty or physical fitness. Once prescribed nurses can adopt health behavior theory-based approaches to helppersons with SCD or MCI to keep exercise schedules. Adherence can be betterfacilitated by establishing rapport between nurses and participants by requesting that caregivers remind participants about their exercise program or by using ways to motivate participants to exercise such as a motivational text message.
Conclusion
As the population with AD increases, activities to maintain or improve cognition of persons at a higher risk of developing AD will be increasingly important to delay the onset of AD. Promotion of aerobic and/or resistance exercise for SCD as well as aerobic, resistance, Tai Chi and multicomponent exercise for MCI may help toward this goal.
Acknowledgements
The authors would like to thank Dr. Ruth Lindquist for hergenerous support.
References
- World Health Organization (2016) World Health Organization media centre: Dementia fact sheet.
- Alzheimer’s Association (2015) Alzheimer's disease facts and figures. Alzheimers Dement 11: 332-384.
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 270-279.
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, et al. (2011) Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 280-292.
- essen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, et al. (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 10: 844-852.
- Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K (2015) Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology 85: 1852-1858.
- Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, et al. (2014) Self-reported memory complaints: Implications from a longitudinal cohort with autopsies. Neurology 83: 1359-1365.
- Ronnlund M, Sundstrom A, Adolfsson R, Nilsson LG (2015) Self-reported memory failures: Associations with future dementia in a population-based study with long-term follow-up. J Am Geriatr Soc 63: 1766-1773.
- Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183-194.
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, et al. (2004) Mild cognitive impairment-beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. J Intern Med 256: 240-246.
- Vega JN, Newhouse PA (2014) Mild cognitive impairment: Diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep 16: 490-498.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, et al. (1999) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: 303-308.
- Citron M (2010) Alzheimer's disease: Strategies for disease modification. Nat Rev Drug Discov 9: 387-398.
- Vidoni ED, Van Sciver A, Johnson DK, He J, Honea R, et al. (2012) A community-based approach to trials of aerobic exercise in aging and Alzheimer's disease. Contemp Clin Trials 33: 1105-1116.
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L (2008) Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 16..
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, et al. (2010) Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med 72: 239-252.
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, et al. (2011) Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med 269: 107-117.
- 18.Barber SE, Clegg AP, Young JB (2012) Is there a role for physical activity in preventing cognitive decline in people with mild cognitive impairment? Age Ageing 41: 5-8.
- Rodakowski J, Saghafi E, Butters MA, Skidmore ER (2015) Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Mol Aspects Med 43: 38-53.
- Strohle A, Schmidt DK, Schultz F, Fricke N, Staden T, et al. (2015) Drug and exercise treatment of Alzheimers disease and mild cognitive impairment: A systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry 23: 1234-1249.
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, (5thedn) DSM-TM. American Psychiatric Publishing, Virginia, USA, pp. 991.
- Garrard J (2011) Health sciences literature review made easy: The matrix method, (3rdedn), Jones & Bartlett Publishers, Ontario, Canada, pp. 212.
- Thomas BH, Ciliska D, Dobbins M, Micucci S (2004) A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 1: 176-184.
- Nagamatsu LS, Chan A, Davis JC, Beattie BL, Graf P, et al. (2013) Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: A 6-month randomized controlled trial. J Aging Res 2013: 861893.
- Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T (2012) Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med 172: 666-668.
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, et al. (2008) Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 300: 1027-1037.
- Lu J, Sun M, Liang L, Feng Y, Pan X, et al. (2015) Effects of momentum-based dumbbell training on cognitive function in older adults with mild cognitive impairment: A pilot randomized controlled trial. Clin Interv Aging 11: 9-16.
- Maki Y, Ura C, Yamaguchi T, Murai T, Isahai M, et al. (2012) Effects of intervention using a community-based walking program for prevention of mental decline: A randomized controlled trial. J Am Geriatr Soc 60: 505-510.
- Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, et al. (2013) The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med 173: 797-804.
- Lam LC, Chan WC, Leung T, Fung AW, Leung EM (2015) Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: A cluster randomized controlled trial. PLoS One 10: e0118173.
- Lam LC, Chau RC, Wong BM, Fung AW, Lui VW, et al. (2011) Interim follow-up of a randomized controlled trial comparing Chinese style mind body (Tai Chi) and stretching exercises on cognitive function in subjects at risk of progressive cognitive decline. Int J Geriatr Psychiatry 26: 733-740.
- Lam LC, Chau RC, Wong BM, Fung AW, Tam CW, et al. (2012) A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc 13: e15-e20.
- Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, et al. (2014) The Study of Mental and Resistance Training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc 15: 873-880.
- Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, et al. (2012) Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: A randomized controlled trial. BMC Neurol 12: 128.
- Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, et al. (2013) A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One 8: e61483.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M (2008) Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 42: 344-351.
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, et al. (2010) Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol 67: 71-79.
- Hildreth KL, Van Pelt RE, Moreau KL, Grigsby J, Hoth KF, et al. (2015) Effects of pioglitazone or exercise in older adults with mild cognitive impairment and insulin resistance: A pilot study. Dement Geriatr Cogn Dis Extra 5: 51-63.
- Young MD, Plotnikoff RC, Collins CE, Callister R, Morgan PJ (2014) Social cognitive theory and physical activity: A systematic review and meta-analysis. Obes Rev 15: 983-995.
- Zheng G, Liu F, Li S, Huang M, Tao J, et al. (2015) Tai Chi and the protection of cognitive ability: A systematic review of prospective studies in healthy adults. Am J Prev Med 49: 89-97.
- Pew Research Center (2013) Cell phone ownership hits 91% of adults.
- Kim BH, Glanz K (2013) Text messaging to motivate walking in older African Americans: A randomized controlled trial. Am J Prev Med 44: 71-75.
- Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, et al. (2015) Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis 48: S171-191.