Journal of Parkinsons disease and Alzheimers disease
Download PDF
Research Article
Amantadine Treatment for Parkinson’s Disease during COVID-19: Bimodal Action Targeting Viral Replication and the NMDA Receptor
Butterworth Roger F*
Department of Medicine, University of Montreal, Canada
*Address for Correspondence: Butterworth Roger F, Professor of Medicine, University of Montreal, Montreal, Qc, Canada 45143 Cabot Trail, Englishtown, NS, B0C 1H0, Canada; E-mail: rb@enceph.com
Submission: 09-June-2020;
Accepted: 30-June- 2020;
Published: 05-July-2020
Copyright: © 2020 Butterworth RF. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Parkinson’s Disease [PD] and COVID-19 share common features
that include age dependency and their association with co-morbidities
such as cardiovascular disease, diabetes and respiratory problems.
Shortness of breath [dyspnea] is a feature of both conditions. Symptoms
of PD are known to deteriorate during systemic infections and
common features of COVID-19 [fever, delirium, stress] may aggravate
tremor, gait and dyskinesias in PD. Parkinsonism is a feature of many
viral encephalatides with associated basal ganglia neuropathology.
Following uptake from the circulation or via the upper nasal transcribial
route, the spike protein of SARS-CoV-2 binds to a host cell protein ACE2
expressed on neurons and neuroglia. Essential host cell proteases such
as Cathepsin L [CTSL] then cleave the spike protein leading to fusion of
viral and host cell membranes and release of the viral genome into the
host cell. Cryo-microscopic studies confirm that SARS-CoV-2 binds with
high affinity to ACE2. High throughput drug screen gene expression
analysis of 466 agents with the potential to down-regulate expression
of CTSL identified amantadine which ranked 5th in efficacy. A link
between viral infection and treatment of PD by amantadine started
serendipitously with the report of a PD patient noting improvement
of tremor and rigidity after treatment with amantadine for influenza
A infection. Amantadine’s beneficial action in PD relates to its ability
to indirectly replenish dopaminergic activity via stimulation of the
NMDA subclass of ionotropic glutamate receptors. An NMDA receptor
antagonist was effective in limiting viral replication with improvement
of neurological symptoms due to infection with HCoV-OC43. The
ability of amantadine to exert beneficial effects in COVID-19 is worthy
of clinical investigation.
Keywords
Parkinson’s disease; COVID-19; SARS-CoV-2 virus; Spike
protein; ACE-2 receptor; Cathepsin L; Neuropathology; Basal ganglia;
Amantadine; NMDA receptor antagonist
Abbreviations
ACE2: Angiotensin Converting Enzyme-2; COVID-19: Coronavirus Disease-2019; CSF: Cerebrospinal Fluid, CNS: Central Nervous System; Cryo-EM: Cryo-Electron Microscopy; CTSL: Cathepsin L; HCoV: Human Coronavirus; MHV: Mouse Hepatitis Virus; NMDA: N-methyl D-aspartate; RT-PCR: Reverse Transcriptase Polymerase Chain Reaction; SARS: Severe Acute Respiratory Syndrome; US-FDA: United States Food and Drug
Administration
Introduction
The severe acute respiratory syndrome Coronavirus-2 [SARSCoV-2] targets multiple organs including the brain resulting in a wide spectrum of neurological conditions. In a retrospective
study from Wuhan, China, neurological manifestations associated with COVID-19 were reviewed in 214 hospitalized patients with laboratory-confirmed diagnosis of SARS-CoV-2 infection [1]. 78 patients [36.4%] manifested neurological symptoms including acute cerebrovascular disease, ataxia, seizure, dizziness, headache, impaired levels of consciousness and skeletal muscle injury. Neurological
symptoms were also reported following a study of 58 patients with SARS-CoV-2 infection where over 80% manifested symptoms of encephalopathy, agitation, confusion and corticospinal tract signs [2]. Although the presence of neurological disorders is not included in the WHO [2020] list of co-morbidities associated with high risk of severe illness from COVID-19, there is evidence to suggest that the presence of such disorders is strongly associated with poor outcome in infected patients [3].
The SARS-CoV-2 genome encodes approximately 25 key proteins
required by the virus in its bid to infect humans and to replicate. The
virus starts by gaining access to the CNS from the circulation or via
the upper nasal transcribial route allowing access to the brain or
peripheral nerve terminals [4]. Then, in common with many other
coronaviruses, the now notorious “spike protein” of SARS-CoV-2
starts its journey by binding to a host cell membrane receptor known
as Angiotensin Converting Enzyme-2 [ACE2]. Brain expresses ACE2
receptors on both neuronal and glial elements in many regions of
the brain including cardio-respiratory centres in the medulla and it
has been suggested that the neuro-invasive potential of the SARSCoV-2 virus plays a role in the acute respiratory failure characteristic
of COVID-19 [5,6]. Following binding of the spike protein to the
ACE2 receptor on the host cell membrane activation of host cell
proteins such as Cathepsin L [CTSL] occurs resulting in cleavage of
the viral spike protein that (Figure 1), in turn, leads to the fusion of
viral and host cell membranes and release of the viral genome into
the cytoplasm of the host cell [7]. Cryo-Microscopic [Cryo-EM]
determination of the SARS-CoV-2 spike confirms that the virus binds
to ACE2 and does so with higher affinity compared to previous SARS
viruses [8].
Disruption of CTSL has the potential to provide the basis for
COVID-19 therapy and this can occur as the result of decreased
expression of CTSL, by inhibition of CTSL enzyme activity or by disruption of CTSL environment resulting from, for example,
increases of pH in the lyposomes [7]. High throughput drug screen
gene expression analysis of 466 agents with the potential to downregulate expression of CTSL identified amantadine which ranked
5th in efficacy. Moreover, since amantadine is also an established
lysosomotropic alkalinizing agent, the possibility of disruption of
lysosomal pH changes was entertained. A number of key lysosomal
pathway genes were found to be down-regulated. Together, these
findings strongly suggest that the mechanism of action of amantadine
is the consequence of its ability to down-regulate CTSL gene
expression coupled with disruption of the CTSL environment caused
by increased lysosomal pH. These mechanisms have the potential to
protect against viral entry and, ultimately, viral replication [7].
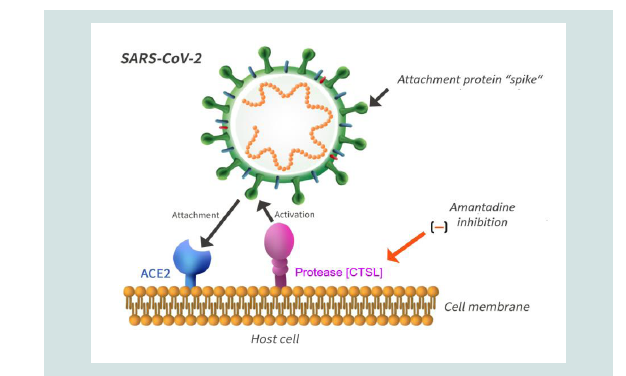
Figure 1: The schematic representation depicts the molecular steps involving
key proteins during invasion of the host cell by SARS-CoV-2.An initial step
involves the binding of the SARS-CoV-2 spike with high affinity to the host
cell membrane protein ACE2, a type 1 membrane protein expressed in lung,
heart, kidney and brain. This is followed by cleavage by host cell proteins, a
key step for viral activation and infection. In the case of SARS-CoV-2, use is
made of the endosomal cysteine protease Cathepsin L [CTSL]. This process
results in fusion of the viral and host cell membranes followed by release
of the viral genome into the cytoplasm of the host cell. Amantadine has the
potential to disrupt the process by down-regulation of the CTSL gene leading
to impaired viral replication.
Parkinson’s Disease [PD] in the COVID-19 era:
PD and COVID-19 share common features including the age
dependency of the two conditions as well as their association with
serious comorbidities such as cardiovascular disease, diabetes and
respiratory difficulties [9]. These observations have drawn attention
to issues relating to the effects of COVID-19 on PD severity, possible
long-term sequelae and effects related to PD care [10]. Conversely,
concerns have been raised relating to the effects of PD on immune
status leading to the possible increased susceptibility of PD patients to
COVID-19 [11]. It is well known that PD symptoms may deteriorate
during systemic infections leading to symptoms ranging from
mild worsening to frank akinetic crisis [12]. Fever is a common
diagnostic symptom in COVID-19 and it has been reported that
delirium and fever may result in subacute motor deterioration in PD
[13]. Moreover, the combination of fever and altered dopaminergic
medication intake has been known to predispose PD patients to
the parkinsonism/hyperpyrexia syndrome, a movement disorder
emergency [14]. Other features of COVID-19 such as stress, fear and
anxiety are known to aggravate tremor, gait and dyskinesias as in PD
[15] and may compromise the efficacy of L-Dopa [16].Enhanced antibody responses to a range of coronaviruses have
been reported in the CSF of PD patients and there is substantial
evidence to suggest that parkinsonism is a feature of several viral
encephalatides with associated PD-type regional neuropathology
[17]. In this latter regard, substantia nigra is known to be susceptible
to damage from H1N1 influenza virus and the coronavirus
MHV-A59 exhibits selective affinity for basal ganglia resulting in
marked postural and locomotor deficits associated with neuronal
cell death and marked gliosis in substantia nigra [18,19]. SARSCoV-2 has been detected in the CSF of two patients with meningitis/
encephalitis and a case of acute necrotizing encephalopathy
associated with cytokine storm in COVID-19 has also been reported
[20-22]. A viral etiology [in whole or in part] for PD raises again
the subject of “post-encephalitic parkinsonism”, a term introduced
following the 1918 pandemic influenza outbreak where the chronic
phase of parkinsonism occurred at various times post-exposure from
immediate to several years or even a decade later [23].
In a study of a cohort of 153 non-demented PD patients without
history of heart or lung diseases, shortness of breath [dyspnea] was
observed in 39.2% of cases accompanied by significantly higher
United Parkinson Disease Rating Scale [UPDRS] scores [24]. It
is likely that the sub-group of PD patients with dyspnea would be
at elevated risk of severe respiratory failure following infection by
SARS-CoV-2.
Amantadine to the rescue?:
The link between amantadine, viral infection and PD started
with the serendipitous observation reported by a 68-year old woman
with moderate-severe PD who, upon taking amantadine for the
management of symptoms of influenza, noted a remarkable remission
in her cogwheel rigidity and tremor; the symptoms reappeared upon
cessation of amantadine. One year later, a clinical trial was conducted
in 163 PD patients treated with amantadine in which the majority
showed significant clinical benefit [25]. Amantadine went on to
receive FDA approval for the treatment of Influenza A. In 2013, using
a robust yeast growth restoration assay together with a sensitive high
throughput screen for the search for inhibitors of the M2 channel
of the influenza virus, 21 active compounds were identified out of
250,000 chemicals and natural products screened; amantadine was
one of the 21 compounds [26].Investigations of the beneficial effects of amantadine against other
viruses have continued apace. Human coronaviruses are established
respiratory pathogens possessed with neuro-invasive and neurotropic
properties. A report published in 2007 described conductance and
binding of amantadine to a pore formed by a lysine-flanked trans
membrane domain of the SARS coronavirus [SARS-CoV] envelope
[E] protein [27]. A subsequent report described the results of studies
of the neuro-invasive human respiratory coronavirus HCoV-OC43,
a strain known to infect human neural cells where it activates
neuroinflammatory and neurodegenerative processes leading to
paralytic disease and motor dysfunctions in virus-infected mice
[28]. Treatment with meantime, a structural analogue of amantadine
resulted not only in attenuation of mortality rates in infected animals;
the treatment also reduced HCoV-OC43 replication in the CNS in
a dose-dependent manner. Both, memantine and amantadine are
potent non-competitive antagonists of the N-Methyl-D-Aspartate [NMDA] subclass of ionotropic glutamate receptors in the brain
(Figure 2). Over-activation of these receptors may result in
excitotoxicity mediated by neural Ca2+ overload leading to neuronal
cell death, a mechanism that has been implicated in the pathogenesis
of neurodegenerative diseases including PD.
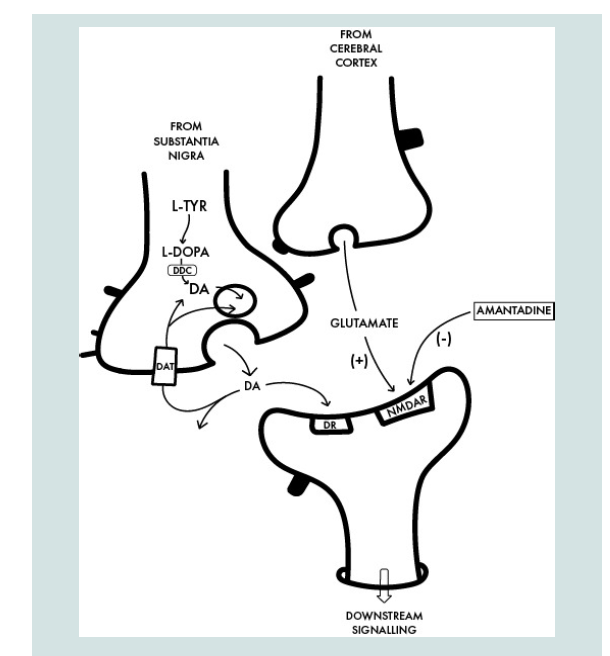
Figure 2: Interface between a dopaminergic [DAergic] nigrostriatal nerve
terminal in which DA is synthetized from L-Tyrosine [L-TYR] via L-DOPA to
DA with a glutamatergic terminal of the cortico-striatal tract and the postsynaptic neuron. The benefit of amantadine for the treatment of the motor
disturbances in PD is attributed to its non-competitive antagonist action on
the post-synaptic NMDA receptor [NMDAR] resulting in the restoration of the
balance between nigrostriatal and corticostriatal inputs in favour of increased
net production of DA. DDC: Dopa Decarboxylase [enzyme for DA synthesis],
DAT: Dopamine Transporter [DA reuptake], DR: Post-Synaptic DA Receptor.
Amantadine is widely used for the treatment of the motor
symptoms of PD and for the control of L-Dopa-induced dyskinesias
[29]. The mechanism of action is predicated on its NMDA receptorantagonist action and there is preliminary evidence of a protective
effect of amantadine in relation to COVID-19 in a study of 5 PD
patients (Figure 2), all receiving L-Dopa, all having tested positive
for SARS-CoV-2 by RT-PCR. None of the 5 PD patients went on to
develop clinical symptoms of COVID-19 and motor function was
unaffected [30]. Given the suggested beneficial effects of amantadine
and possibly other members of the adamantane group of compounds
against coronaviruses, repeated appeals have been made for the
repurposing of these agents for the treatment of COVID-19 [31].
Conclusions and Future Prospects
The review focuses on the cerebral consequences of COVID-19
in the light of the current pandemic that, at the time of submission
of this manuscript had infected over 10 million people worldwide
with attendant fatality rate in excess of 500,000. In addition to patient
age and of comorbidities such as cardiovascular disease, diabetes and respiratory disorders it may now be appropriate for the presence of
chronic neurological disorders be included in the high-risk group for
severe COVID-19 according to modified WHO guidelines.
Amantadine was approved by US-FDA since 1968 as a prophylactic
agent for influenza and more recently for the treatment of PD and its
associated dyskinesias. Importantly, in the high throughput screening
study in which amantadine was noted to down-regulate the expression
of the host-cell protease CTSL, the effective dose of amantadine was
within one order of magnitude of the drug’s clinical pharmacokinetic
profile for the treatment of PD and could therefore conceivably be
employed within current labelling guidelines [7]. There is currently
very little information relating to side effects of amantadine in the
context of COVID-19 therapy; side effects in PD patients treated
with amantadine are relatively mild consisting of confusion, blurred
vision, foot edema and constipation. Hallucinations have occasionally
been described following abrupt discontinuation of amantadine. On a
related topic, there is currently no published literature relating to the
evolution of resistance to amantadine of the SARS-CoV-2 virus but,
in view of the established amantadine resistance in the case of other
RNA viruses such as influenza-A, it will be essential that laboratory
surveillance be performed in a timely manner [31].
Amantadine has the capacity to interfere with molecular
mechanisms implicated in the replication of SARS-CoV-2 resulting
in reduced viral load as well as its associated disease severity and
progression. Concomitantly, amantadine has the potential, by virtue
of its NMDA receptor antagonist action, to restore the dopaminergic
deficit in PD (Figure 2). Several appeals have been made for the
initiation of appropriate clinical trials on the use of amantadine for
the treatment of COVID-19 [31-34].
Acknowledgement
Research from the author’s Unit including work on
Parkinsonism and costs of publication of original articles and
reviews was in part funded over the last two decades by The
Canadian Institutes of Health Research (CIHR) and The Canadian
Association for Study of The Liver (CASL). The author is grateful
to Mr Jonas Eric Pilling for the design of Figures 1 and 2.