Journal of Pediatrics & Child Care
Download PDF
Case Report
An Unintended Case-Control Study: Corticosteroid Growth Restriction in a Monozygotic Twin
Alexandr Magder EL and Jerry Zimmerman
1 Albany Medical Center, 363 Ontario St #C101, Albany, New York, USA,
2Seattle Children’s Hospital, New York, USA
2Seattle Children’s Hospital, New York, USA
*Address for Correspondence:Alexandr Magder EL, Albany Medical Center, 363 Ontario
St #C101, Albany, New York, USA, Tel: +1-518-772-8097, Email: magdera@amc.edu
Submission: 23 May 2023
Accepted: 15 June 2023
Published: 16 June 2023
Copyright: © 2023 Alexandr Magder EL, et al. This is an open
access article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution,
and reproduction in any medium, provided the original work
is properly cited.
Keywords: Asthma; Growth/Developmental milestones; Inhaled corticosteroids
Abstract
Medication errors are an important cause of morbidity and
mortality in children, occurring roughly every 8 minutes for children
under 6. Among all medical errors, medication dosing errors are
very common. In this context, we present the case of amonozygotic
twin girl with severe, persistent asthma, who erroneously received
a double dose of nebulized budesonide (2 mg/day) for a period
of 23 months. Although the patient’s height and weight tracked
along the 50thpercentile before starting budesonide, these growth
measures fell to the 5th and 10thpercentile respectively following the
initiation of this prescription error, despite having improved asthma
control over this period. In contrast, during the same time period, the
patient’s twin sister experienced normal growth along the 50th centile.
Furthermore, this patient experienced long-term harm: three years
after discontinuing the erroneous budesonide dose, growth did not
return to the 50th percentile and a DEXA scan documented persistent
osteopenia, a known side effect of corticosteroids.
This case highlights key considerations to reduce harm using ICS medications: First, any changes in growth trajectory should prompt a review of inhaled corticosteroid medications being used. Second, corticosteroid medications should be reviewed at any hospital visit. The use of clinical standard work pathways in primary care and the emergency department can help to standardize medical management and reduce the risk of medical errors. Finally, minimal effective doses of corticosteroids (and all medications) should be prescribed.
This case highlights key considerations to reduce harm using ICS medications: First, any changes in growth trajectory should prompt a review of inhaled corticosteroid medications being used. Second, corticosteroid medications should be reviewed at any hospital visit. The use of clinical standard work pathways in primary care and the emergency department can help to standardize medical management and reduce the risk of medical errors. Finally, minimal effective doses of corticosteroids (and all medications) should be prescribed.
Abbreviations
Inhaled corticosteroids (ICS), Dual-energy X-ray absorptiometry
(DEXA), Medication taken inhaled (INH), Medication taken as
needed (PRN), Metered dose inhaler (MDI), Actuation (ACT)
Introduction
It is estimated that in the United States, a medication error
occurs every 8 minutes for a child under 6 years of age [1]. Following
medication omission, the second most commonly cited medication
error is incorrect dosage [2]. Here we present the case of a prepubertal
girl who received an unintended double dose of nebulized
corticosteroid for 23 months and experienced substantial growth
restriction as compared to her monozygotic twin.
While inhaled corticosteroids (ICS) are recommended as firstline
therapy for the control of asthma and have a clear benefit in
reducing related morbidity and mortality, [3] chronic ICS use has
the potential to reduce growth velocity [4-6]. While ICS doses above
100 mcg offluticas one or equivalent result in minor improvements
to asthma control, adverse impact on growth restriction is dose-dependent,
with minimal effects at therapeutic doses [4].
This case report ascertains the need for regular medication review and robust practices to monitor both benefit as well as potential risks of long-term corticosteroids administered to pre-pubertal children.
Case Presentation
This is the case of a monozygotic twin girl, born at 34weeks, 4
days estimated gestational age, who was evaluated at the age of 3.5
years for severe, uncontrolled asthma in a respiratory clinic at a
Canadian tertiary pediatric centre. The referral had been requested
one-month prior by an emergency department physician following a
severe asthma exacerbation in this toddler. At the time of her initial
clinic assessment, the patient’s parents reported a series of episodes
consisting of acute-onset shortness of breath in the absence of viral
symptoms or identified environmental triggers, which responded
to nebulized salbutamol. Despite a 1-month tapered course of oral
prednisolone, initially 2mg/kg/day, the parents reported that the
patient required a rescue dose of inhaled salbutamol every four
hours to control her symptoms. At this time the patient’s regular
medications included salbutamol metered-dose inhaler (MDI) as
required, vitamin D6000 IU by mouth once daily, montelukast 4mg
by mouth at bedtime, and mometasone nasal spray 50mcg once daily.
The therapeutic outcome of this visit was addition of a fluticasone/
salmeterol 25/125 mcg/actuation (ACT) MDI dosed twice daily.
A complicating factor in the control of this patient’s asthma was
an underlying diagnosis of mannose-binding lectin (MBL) deficiency.
This condition was diagnosed after a referral to immunology following
a series of lower respiratory tract infections and recurrent otitis media
requiring antibiotic treatment from the age of two years.
In comparison, the patient’s monozygotic twin sister had two
presentations to the emergency department for laryngotracheo
bronchitis between 2 -3 years of age, she did not have a history of
recurrent infections, persistent asthma symptoms, and was never
diagnosed with MBL deficiency. The parents did however report
the twin sister was referred to pulmonology for recurrent symptoms
consistent with reactive airway disease (RAD) and was prescribed inhaled fluticasone 50 mcg (roughly equivalent to 95 mcg budesonide) [7], dosed once daily to be taken with exacerbations. This medication
was used infrequently (fewer than 30 days over the year) and was
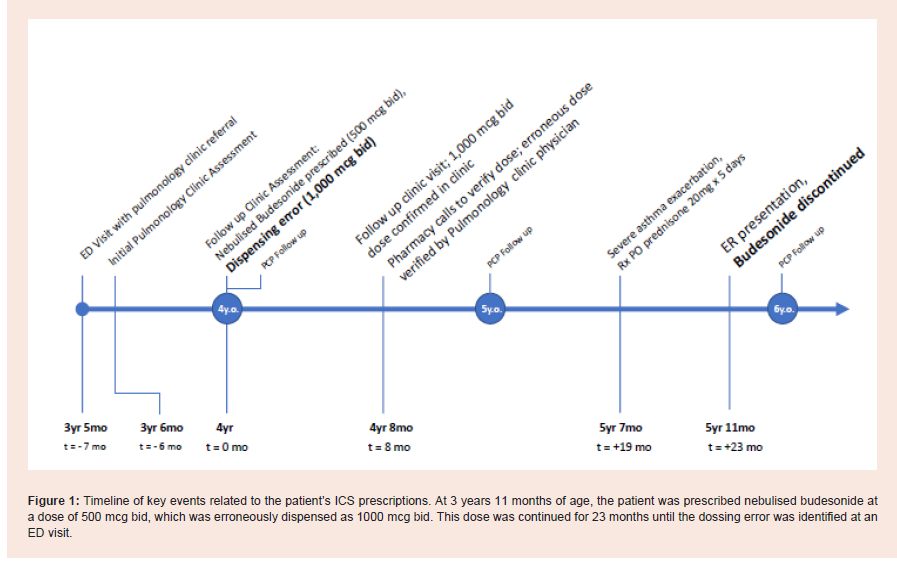
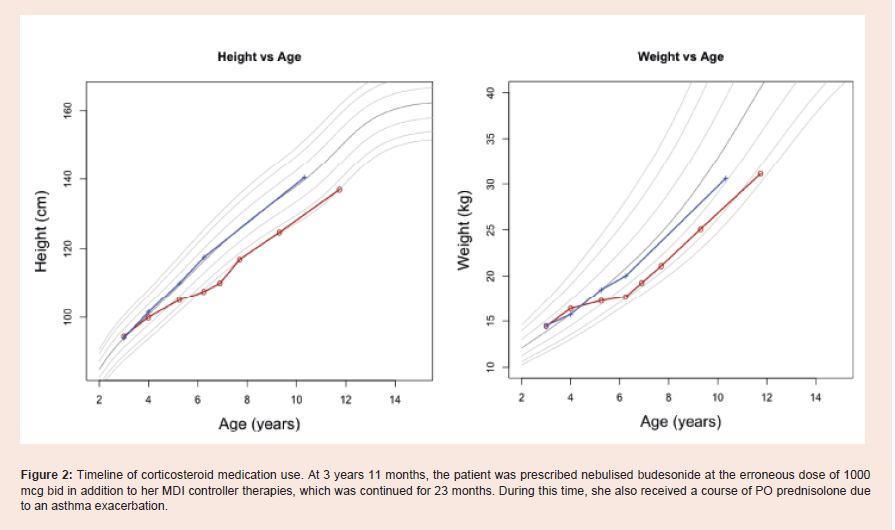
discontinued after 6 months [Figure 1 and 2 ].
Between clinic visits, which occurred annually with the patient’s
pediatrician and roughly every 6 months with the pediatric
pulmonology clinic, the patient continued to experience asthma
exacerbations, with roughly one ED visit per month, one of which was
severe enough to warrant a one-week prescription of PO prednisone
dosed 2mg/kg/day.
At the first follow-up visit following this enteral course of
prednisone, the patient’s height and weight tracked along the 50th
percentile. At this time her medications included fluticasone/
salmeterol 125/25mcg/ACTMDI two inhalations twice daily, lansoprazole
15mg by mouth once daily, nebulised ipratropium bromide
every 6 hours as needed, and salbutamol MDI 100 mcg every 4 hours
as needed taken once a day when well.
Due to the recurrent nature of her asthma exacerbations, a
decision was made to start nebulized budesonide at a dose of dosed
500 mcg twice daily when the patient was 3 years 11 months old.
However, the pharmacy erroneously dispensed nebulised budesonide
at a dose of 1000 mcg twice daily, double the highest budesonide
dose recommended by the FDA [8]. After 8 months, the pharmacy
noticed the error and contacted the prescribing physician. Despite a
correction from the prescribing physician, the pharmacy continued
to dispensetheerroneous higher dose of 1000 mcg twice daily.
Budesonide 2000 mcg daily was continued for a period of 23 months from 4 to 6 years of age. During this time, the patient remained on
fluticasone/salmeterol 125/25mcg MDI twice daily until 4 years 8
months of age, when this was exchanged in favor of mometasone/
formoterol 200mcg/5mg/ACT twice per day. During this time, she
experienced improved asthma symptom control per parental report,
with a single substantial exacerbation at 4 years 6 months that
required a 5th day course of oral prednisolone, dosed 1.2mg/kg/day.
from 4 to 6 years of age. During this time, the patient remained on
fluticasone/salmeterol 125/25mcg MDI twice daily until 4 years 8
months of age, when this was exchanged in favor of mometasone/
formoterol 200mcg/5mg/ACT twice per day. During this time, she
experienced improved asthma symptom control per parental report,
with a single substantial exacerbation at 4 years 6 months that
required a 5th day course of oral prednisolone, dosed 1.2mg/kg/day.
At the age of 6 years 0 months, 23 months after starting nebulised
budesonide at the erroneous dose, the patient presented to the
emergency department following a one-day history of lethargy,
vomiting, loss of balance, frontal headache, and poor oral intake,
identified as concerning for adrenal failure per the emergency
department discharge summary. A review of the patient’s medications
was performed at that time, which revealed the underlying prescription
error. On discharge, the nebulised budesonide discontinued entirely.
Within one month of the cessation of the inhaled budesonide,
a growth chart assessment indicated that the patient’s height and
weight tracked at the 5th and 10th centiles respectively. In contrast, on
this date, the patient’s identical twin tracked at the 50th percentile for
both height and weight. A DEXA scan of the patient revealed bone
mineral density loss in the lumbar spine, corresponding to an agematched
Z-score of -2.5.
Three years after discontinuing budesonide, the patient was
diagnosed with steroid-induced osteoporosis with at Z-score of -1.7.
At this time, her height and weight corresponded to the 5th and 13th
centiles respectively. In contrast, her twin sister continued to track
along the 50th centiles for height and weight. Between the age of 4-5, while receiving 2000 mcg of budesonide daily, the patient experienced eight asthma exacerbations resulting in emergency department visits.
However, between the ages of 5-11 years, the patient experienced at
most 1 exacerbation per year, while undergoing accelerating growth
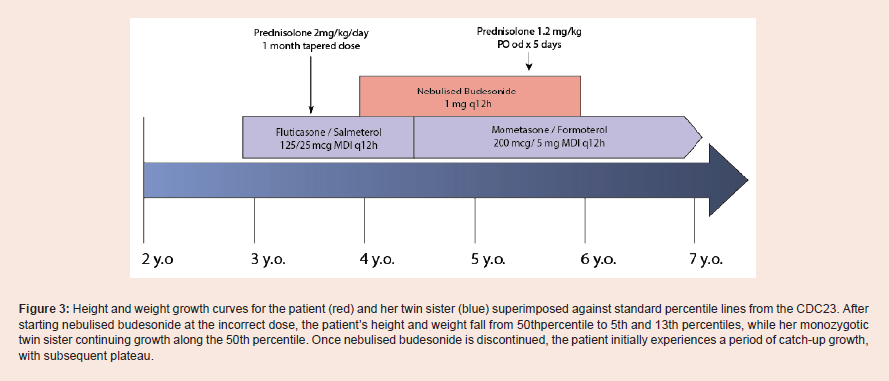
velocity [Figure 3].
Figure 1: Timeline of key events related to the patient’s ICS prescriptions. At 3 years 11 months of age, the patient was prescribed nebulised budesonide at a dose of 500 mcg bid, which was erroneously dispensed as 1000 mcg bid. This dose was continued for 23 months until the dossing error was identified at an
ED visit.
Figure 2: Timeline of corticosteroid medication use. At 3 years 11 months, the patient was prescribed nebulised budesonide at the erroneous dose of 1000 mcg bid in addition to her MDI controller therapies, which was continued for 23 months. During this time, she also received a course of PO prednisolone due to an asthma exacerbation.
Figure 3: Height and weight growth curves for the patient (red) and her twin sister (blue) superimposed against standard percentile lines from the CDC23. After starting nebulised budesonide at the incorrect dose, the patient’s height and weight fall from 50thpercentile to 5th and 13th percentiles, while her monozygotic twin sister continuing growth along the 50th percentile. Once nebulised budesonide is discontinued, the patient initially experiences a period of catch-up growth,
with subsequent plateau.
Discussion
Several factors implicate the patient’s corticosteroid prescription
in her growth restriction noted between 4-6 years of age. Most
importantly, the patient’s growth velocity notably increased once
the incorrect budesonide prescription was discontinued. In addition,
osteopenia noted in the lumbar spine is a typical adverse effect of longterm
corticosteroid use [9-11]. However, lansoprazole, prescribed in
the setting of high dose corticosteroid administration, may have also
contributed to the osteopenia [12]. Finally, the substantial difference
in the growth curves of the two girls, with the patient falling to the
5th and 13th percentiles for height and weight, while her monozygotic
twin sister continuing growth along the 50th centiles, provides a strong argument that differences in inhaled corticosteroid dosing
played a major role[Figure 3]. While the patient was administered
2000 mcg inhaled budesonide and 400 mcg fluticasone (equivalent
to 900 mcg budesonide) [7] per day, her twin sister received 50 μg of
inhaled fluticasone (equivalent to 95 mcg budesonide) [7] per day for
fewer than 30 days annually.
The most compelling alternative reason for the patient’s growth
restriction is the patient’s poorly controlled asthma. The patient’s
MBL deficiency should be considered in this context, as it has been
postulated this condition may contribute to airway inflammation and
thus increase the risk of developing asthma [13]. However, the patient
experienced poorly controlled asthma for two years before the start of
the budesonide prescription at the erroneous dose, during which time
the patient’s growth velocity closely followed that of her sister and
remained along the 50th percentile. In addition, rather than growth
velocity increasing following improved symptomatology between 5-6
years of age, the patient continued to experience lower than expected
growth velocity, falling from the 50th centile to the 5th centile for
height.
While data remains conflicting regarding the effects of ICS
medications on linear growth at regular dosing, a 2014 Cochrane
review suggested low or medium-dose ICS use was associated with
a reduction in linear growth velocity of 0.48 cm/year, particularly in
the first year of ICS therapy [14]. In addition, it should be noted some
studies have reported a decrease in final adult height in the range of
1.2cm on average, even at moderate ICS dosing [15].Effects on growth
have been reported to be dose dependent, and are most significant
above a dose of 0.4mg/day, a threshold far surpassed by this patient
over a period of two years [15]. By age 6, the patient’s height was
nearly 10cm less than her twin sister, which further widened to 15cm
at 10 years of age, suggesting a long-lasting difference in final adult
height is likely.
This case highlights the importance of regular review of
medications and assessment of growth for children with asthma,
particularly those prescribed corticosteroids [16]. In many cases,
inhaled corticosteroid dose responses plateau at 200 mcg fluticasone
per day or equivalent, and few children benefit from dose increases
beyond 500 mcg day [17] (equivalent to 1000 mcg budesonide) [7].
Thus, dosing beyond this threshold was unlikely to provide additional
symptom control, which explains why the patient continued to
experience severe asthma exacerbations.
From a systems perspective, there are a number of key drivers
that contributed to this medication error: 1) faltering growth should
have raised concern with the patient’s pediatrician; 2) at the time of
the error, the pulmonology clinic used paper charts which did not
display warnings for doses; 3) the pharmacy should have verified
the dose immediately, rather than 8 months later; and finally, 4) the
improvement in the patient’s asthma control should have prompted a
review of her corticosteroid medications.
Implementation of asthma clinical standard work pathways
represent potential solutions to increase adherence to NIH asthma
guidelines [18]: the “Primary Care Pathway for Childhood Asthma”
[19] is an example currently being studied for primary care, and
many children’s hospitals employ clinical pathways to standardize
asthma care in their emergency department and following hospital
discharge [20]. In hospital, mandatory reconciliation of admission/
discharge medications can identify errors or concerns [21]. These
medication safety exercises and standardized treatment algorithms
may have been particularly useful for this patient and may have
prevented harm.
In summary, while inhaled corticosteroids remain the most
effective therapy available for maintenance treatment of childhood
asthma, it is imperative that clinicians use the lowest-effective
corticosteroid dose for treating asthma (or other corticosteroidresponsive
conditions). Importantly, growth charts should be regularly
reviewed in the context of chronic corticosteroid administration, and
a full medication review should occur if growth begins to falter [22].
Contributors’ Statement Page:
Dr Magder obtained the consent from the patient’s family for
publishing, created the figures, and drafted the initial manuscript.Dr Zimmerman supported the drafting of the initial manuscript
by providing expert advice on the interpretation of clinical data,
supported the creation of the figures and revised the initial manuscript
for accuracy.
Dr Magder and Dr Zimmerman critically reviewed and edited the
revised manuscript.
Ethics approval and consent to participate:
Approval granted by the Royal College of Surgeons Research
Ethics Board.Acknowledgements
We would like to thank the family for giving us the privilege to
learn from this case study. We understand how difficult this period in
their lives has been, and we hope this case will help prevent similar
errors in the future.