Journal of Pharmaceutics & Pharmacology
Download PDF
Research article
Preparation and Evaluationof Ibuprofen Solid Dispersion Tablets with Improved Dissolution and Less Sticking Using Porous Calcium Silicate
Takahashi K* , Hirai N and Takatani-Nakase T
- Department of Pharmaceutics, School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women’s University, Hyogo, Japan
*Address for correspondence: Koichi Takahashi, Department of Pharmaceutics, School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women’s University, Hyogo, Japan, Tel: +81 798459943; Fax: +81 798 45 9943; E-mail: koichi@mukogawa-u.ac.jp
Citation: Hirai N, Takatani-Nakase T, Takahashi K. Preparation and Evaluation of Ibuprofen Solid Dispersion Tablets with Improved Dissolution and LessSticking Using Porous Calcium Silicate. J Pharmaceu Pharmacol. 2018; 6(1): 8.
Journal of Pharmaceutics & Pharmacology | ISSN: 2327-204X | Volume: 6, Issue: 1
Submission: 10 September 2018| Accepted: 24 September 2018 | Published: 03 October, 2018
Copyright: © 2018 Hirai N, et al. This is an open access articledistributed under the Creative Commons Attribution License, whichpermits unrestricted use, distribution, and reproduction in any medium,provided the original work is properly cited.
Abstract
The aim of this study was to prepare and evaluate Ibuprofen (IBU) solid dispersion tablets with improved dissolution and less sticking using Porous Calcium Silicate (PCS). Solid dispersion granules were prepared using water-based solid dispersion and water-retained PCS with the wet granulation method and a high-speed agitation granulator. We attempted to use four binders [Hydroxypropylcellulose (HPC), dextrin, maltitol, and xylitol] to prepare the granules; however, HPC could not be used. After 1h of continuous tableting using a rotary machine, sticking was estimated by a visual inspection of all tablets and photographs of the upper punches with a digital camera. All prepared tablets demonstrated quick disintegration and dissolution. From the results of DSC and PXRD studies, IBU may exist in an amorphous form in the adsorption solid dispersion and the granules. From FT-IR studies, IBU appears to interact with PCS through a salt formation between the COO-groups of IBU and the Ca2+ ions of PCS. To investigate differences in binders on the sticking problem, DSC, NIR spectra, and bulk density were measured. From these results, we speculated that the primary localization of the specific binder in the PCS pores may differ among binders, with this localization affecting the sticking property.
Keywords
Ibuprofen; Poorly water-soluble drug; Porous calcium silicate; Wet granulation method; Sticking; High-speed mixer
Introduction
Solid dosage forms are defined as drug delivery systems that are presented as solid dose units [1]. Tablets are the most popular and preferred drug delivery vehicles. Thus, tablets represent the first choice of dosage form for new drug candidates. Tablets are manufactured using a high-speed rotary tableting machine; however, sticking or picking is a common problem encountered during tablet compaction. Sticking is a phenomenon where part of the tablet attaches to the surface of the punch or detaches, resulting in a clouding or an indentation of the tablet surface. It has been reported that sticking may be due to excess water in the powder [2], an insufficient amount of lubricant [3], an excessive amount of binder [4], melting of drugs with low melting points [5], or punch surface conditions [6,7].
Ibuprofen (IBU) is often administered as a solid oral dosage form with a high dose (200-800 mg) [8]. However, IBU displays poor compaction behavior and has a high prevalence for sticking [9]. Aoki and Danjo suggested that the degree of sticking of a particular IBU formulation increases with increasing compression speed and decreases with increasing compression force [10]. This may be attributed to the low melting point of IBU, which is 72 °C-75 °C [6,11]. To prepare tablets, the direct compression, dry granulation, and wet granulation methods are well known. To obtain high economic efficiency, the direct compaction method is typically the preferred production method [12,13]. Several studies have reported methods to improve the tableting behavior of IBU [9,14,15]. However, as IBU has poor compressibility, it is difficult to prepare IBU tablets containing the proper drug content (about 30%) using the direct compression method [16]. Thus, IBU formulations often have to begranulated [9,17]. Recently, the nanocoating of IBU powder particles with various excipients, such as fumed silica or magnesium stearate [18,19], have been reported. In these studies, powder flowability and tablet ability were improved, but the tendency for sticking was not investigated.
IBU is a type of Biopharmaceutics Classification System class II drug (aqueous solubility<0.1 mg/ml) [20]. Various techniques, such as particle size reduction, crystal habit modification, complexation, solubilization, solid dispersion in carriers, and salt formation, have been employed to improve the aqueous solubility of class II drugs [21]. Solid dispersion is achieved by spray-drying [22,23], hot-melt extrusion [24,25], supercritical fluid [26], and cryogenic freezing technologies [27]. However, these technologies are limited in theirability to scale-up [28-31].
The solvent evaporation method is a manufacturing technique used to prepare solid dispersion [32]. The solid dispersion of a drug is generally prepared by dispersion in a water-soluble carrier. Polyethylene glycols, Hydroxypropyl Methylcellulose (HPMC), Poloxamer, and Polyvinylpyrrolidone (PVP) are widely used as solid dispersion carriers because of their strong hydrophilic properties and ability to form molecular adducts with many compounds [31,33]. Recently, the preparation of surface solid dispersion has been reported [34,35]. Unlike conventional solid dispersion, the carriers used in surface solid dispersion are water-insoluble and porous materials with hydrophilic properties. Silica gel and calcium silicate have large surfaces attributed to their highly porous structure. Several studies have reported methods to improve the solubility of poorlywater-soluble drugs [36-39]. Porous Calcium Silicate (PCS) possesses many interparticles (12 µm) and intraparticle (0.15 µm) pores on its surface, and has been used as a liquid absorber and a compressive adjuvant of powders for tableting [40]. PCS is also used as a carrier of solid dispersion for improving the dissolution of poorly water-soluble drugs [38-41]. We previously reported the development of solid dispersion tablets by the simple and manufacturable wet granulation method using PCS [42,43].
The purpose of the present study was to obtain a solid dispersion IBU product with improved solubility and less sticking. The influence of drug-PCS ratios on the interaction between IBU and PCS was also studied. It is difficult to determination whether the drug or the excipient is present as a crystalline particulate or an amorphous particulate dispersion. Therefore, Differential Scanning Calorimetry (DSC), Fourier-Transformed Infrared (FT-IR) spectroscopy, Fourier-Transformed near Infrared (FT-NIR) spectroscopy, and X-ray diffractometry were employed to determine how the drug isdispersed within the matrix and to study the nature of the molecular interactions in the IBU-PCS system.
Materials and Methods
Materials
Ibuprofen (IBU) and PCS (Fluorite® RE) were purchased from Nissin Pharma Inc. (Tokyo, Japan) and Tomita Pharmaceutical Co. Ltd. (Tokushima, Japan), respectively. Hydroxypropyl Cellulose (HPC-L, HPC), xylitol, and dextrin were obtained from Nippon Soda Co. Ltd. (Tokyo, Japan), Towa Chemical Industry Co. Ltd. (Osaka, Japan) and Mitsubishi-chemical foods corporation (Tokyo, Japan), respectively. Crospovidone and magnesium stearate were purchased from BASF Japan Co. Ltd. (Tokyo, Japan) and TAIHEI Chemical Ind. Co. Ltd. (Osaka, Japan), respectively. Other reagents were of analytical grade and were used without further purification.
Preparation and evaluation of granules using the wet granulation method
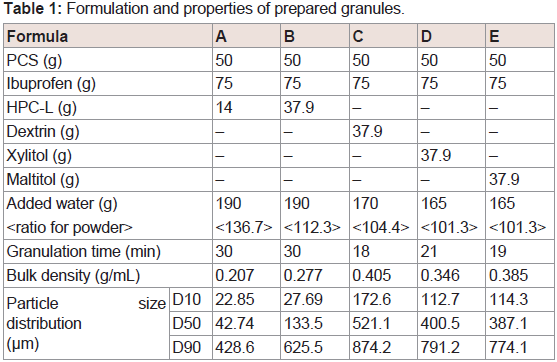
Water was added to PCS in the vessel of the granulating machine and mixed for 5 min with the granulator at 200 rpm with an agitator and 3000 rpm with a chopper (High-Speed Mixer LFS2,EARTHTECHNICA Co. Ltd. Tokyo, Japan). IBU was then added to the water-retained PCS and mixed for 15 min. Binder was then added to the mixture to prepare the granules, and the end-point of the granulation was determined visually. After drying at 70 °C for 12 h, the granules were pulverized in a speed mill (Okada Seiko Co. Ltd. Tokyo, Japan). Particle size distribution of the obtained granules was measured by laser diffraction and the scattering method using a drymodule (LS 13 320, Beckman Coulter, Tokyo, Japan).
The bulk density and tapped density of granules were measured according to method 1 (measurement in a graduated cylinder) of the determination of bulk and tapped densities in Japanese Pharmacopoeia 17. Compressibility (%) was calculated by thefollowing equation:
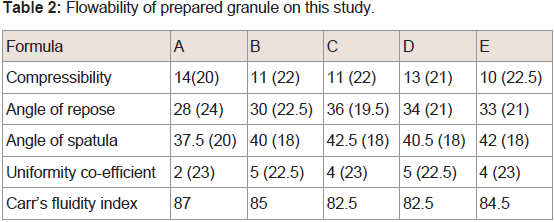
Carr’s fluidity index was calculated with the point scores as previously described [44]. The angle of repose was measured using a protractor for the heap of granules formed by passing 10.0 g of the sample through a funnel at a height of 8 cm from the horizontal surface. The angle of spatula was measured using a protractor and a steel spatula with a blade. The spatula was inserted to the bottom of the heap that was carefully built by dropping the granules through a funnel at a height of 8 cm from the horizontal surface. The spatula was then vertically withdrawn, and the angle of the heap formed on the spatula was measured as the angle of spatula.
The granule size parameter (e.g. D10, D50, D60 and D90) was measured using laser diffraction and the scattering method. Uniformity coefficient was calculated from the following equation:
Preparation of the adsorption solid dispersion and physical mixture
Water (100.0 g) was added to PCS (50.0 g) and mixed for 5 min. IBU (75.0 g) was then added to the PCS-retained water and mixed for 15 min with the granulator at 200 rpm and with an agitator at 3000 rpm with a chopper. Adsorption Solid Dispersion (ASD) was prepared after drying the powder at 70 °C for 12 h in a drying oven. The Physical Mixture (PM) was prepared by mixing IBU (7.5 g) and PCS (5.0 g).
Preparation and evaluation of tablets
Granules were mixed with crospovidone and magnesium stearate at 25 rpm for 10 min using a V-model mixer (S-5, Tsutsui Scientifical Ind. Tokyo, Japan). These mixtures were then compressed using a rotary tableting machine (EX-SS15, Hata-tekkosho Co. Ltd. KyotoJapan) with 10-mm diameter bi-convex punches at a rotating speed of 25 rpm. All batches of tablets weighed 400 mg and the target compression load for each batch was about 500 kg.
The bulk densities of the granules were measured according to the method 1 (measurement in a graduated cylinder) to determine the bulk and tapped densities in Japanese Pharmacopoeia 17 (JP 17). To estimate the localization of IBU and binder in PCS, bulk densities ofthe adsorption samples were measured with the same method.
The hardness of the tablets was measured by the diametric compression method with a PC-30 (Okada seiko Co. Ltd. Tokyo, Japan). Ten tablets were tested in each batch, and mean values were calculated. The disintegration times of six tablets from each batchwere measured individually in purified water at 37 °C±0.5 °C using a JP 17 apparatus (NT-2HS, Toyama Sangyo Co. Ltd. Osaka, Japan), and mean values were calculated.
After 1 h of continuous tableting, the sticking problem was estimated by two methods. One was the visual inspection of all tablets, while the other was the examination of photographs of the upper punches with a digital camera.
In vitro dissolution
In vitro dissolution tests were performed using a JP 17 apparatus (NTR-6200, Toyama Sangyo Co. Ltd. Osaka, Japan). One tablet containing IBU was placed in double distilled water (900 mL) at 37 °C±0.5 °C at 50 rpm. The amount of dissolved IBU was determined spectrophotometrically using a double beam spectrophotometer (UV-1280, Shimadzu Corporation, Kyoto, Japan) at 264 nm. The dissolution studies were carried out in triplicate for each batch, and the mean values were calculated. Ten tablets were ground to estimate the IBU content in each tablet, with the ground powder equivalent to 10 mg of IBU, which was accurately weighed and dissolved in methanol. After suitable dilution with methanol, IBU was analyzed by a spectrophotometer.
Powder X-Ray Diffraction (PXRD)
PXRD analysis was performed with Cu K-ALPHA1 radiation, a voltage of 40 kV, and a current of 200 mA (RINT-2000, Rigaku Corporation, Tokyo, Japan). The scan rate was 5°/min over a 2θ range of 5-70°, with a sampling interval of 0.02°.
Fourier-Transformed Infrared (FT-IR)
FT-IR studies were carried out using a FT/NIR-IR spectrometer (PerkinElmer Frontier, Perkin Elmer Japan Ltd. Kanagawa, Japan). Samples were directly measured using the ATR module. Each sample reading averaged 16 individual spectra at a resolution of 4 cm−1 and was scanned over the region 400-4000 cm−1 wave numbers.
Fourier-Transformed Near Infrared (FT-NIR)
FT-NIR measurements were performed using a FT-IR/NIR spectrometer with an InGaAs detector. Samples were directly measured using a Reflectance Accessory (NIRA). Each sample reading averaged 32 individual spectra at a resolution of 4 cm−1 and was scanned over the region 4000-10000 cm−1 wave numbers.
Differential Scanning Calorimetry (DSC)
DSC analyses were performed using an automatic thermal analyzer (DSC-60 Plus, Shimadzu Corporation, Kyoto, Japan) and indium standard for temperature calibrations. Holed aluminum pans were employed in the experiments for all samples and an empty pan, prepared in the same way, was used as a reference. Samples (3-6 mg) were sealed in the aluminum pans, and heating curves were recorded using a constant heating rate of 5 °C /min from 25 °C to 200 °C.
Results and Discussion
Optimization of PCS:IBU and estimation of interaction between PCS and IBU
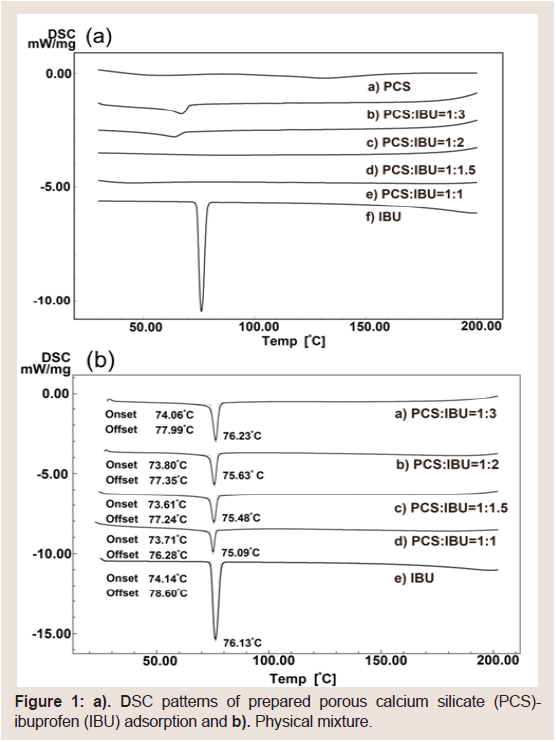
In order to obtain the optimum PCS:IBU ratio for the preparation of solid dispersion tablets, the DSC thermograms of different IBU adsorptions with PCS at different PCS:IBU ratios (1:1, 1:1.5, 1:2 and 1:2.5) were measured (Figure 1). IBU exhibited a melting endotherm at 76 °C. An endothermic peak due to the melting of the drug was observed in the samples of PCS:IBU (1:2 and 1:2.5). However, this peak was not observed in samples of PCS:IBU (1:1 and 1:1.5). These findings indicate a certain degree of IBU crystallinity loss in the samples. This loss of crystallinity may be due to several reasons, with the main one being that the dilution effect resulted from the increase in PCS. Accordingly, the crystalline IBU in the PM was determined to further investigate the dilution effect. Endothermic peaks wereobserved in all PMs; areas under the melting curves decreased in the PMs with increased amounts of PCS compared with IBU (Figure 1). From these results, IBU may be present in an amorphous state in samples of PCS: IBU (1:1 and 1:1.5).
Figure 1: a) DSC patterns of prepared porous calcium silicate (PCS)- ibuprofen (IBU) adsorption and b). Physical mixture.
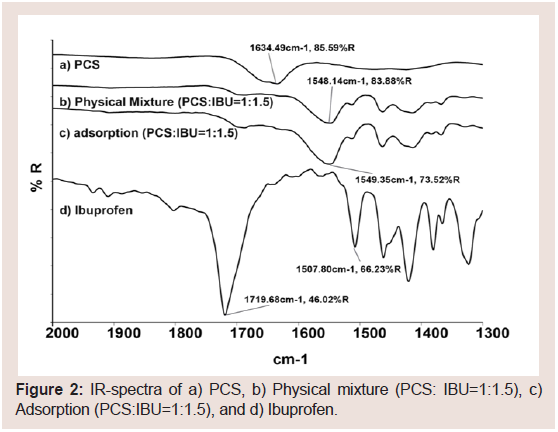
To investigate the potential interactions between IBU and PCS, FT-IR spectra of PCS with and without IBU loading were measured and are shown in (Figure 2). IBU contains an acid carboxyl group, and the IR spectrum of pure IBU showed a characteristic peak at 1720 cm−1 (acid C=O stretching). In contrast, the adsorption sample (PCS:IBU = 1:1.5) showed a new absorption at 1549 cm−1. This peak shift was also observed in the PM. Guo et al. investigated the interaction between calcium silicate hydrate and IBU [45], and reported that the Si-O stretching vibration at 975 cm−1 of calcium silicate hydrate shifts to 1083 cm−1 , the acid C=O stretching at 1724 cm−1 of IBU shifts to 1560 cm−1, and the C=O stretching vibration of IBU does not shift. The authors suggested that chemical interactions occur between the -COOH groups of IBU and -Si-O-Ca groups of PCS. From these results, IBU may interact with PCS through a salt formation between the COO− groups of IBU and the Ca2+ ions of PCS. This interaction was observed not only in ASD but also in PM.
Figure 2: IR-spectra of a) PCS, b) Physical mixture (PCS: IBU=1:1.5), c) Adsorption (PCS:IBU=1:1.5), and d) Ibuprofen.
Preparation of PCS granules and tablets
Because of the low density of PCS it is difficult to use in the manufacture of solid formulations. To prepare tables, it is essential that granules with a large density containing PCS are prepared. Hirai et al. and Fujimoto et al. have suggested that the wet granulation method [42,43,46], using saccharide and xylitol, is effective for improving the PCS property of a small specific gravity. In this study, IBU granules were prepared using four binders (HPC, dextrin, xylitol, and maltitol), and the formulations of the granule, granulation times, bulk densities, and particle size distribution were determined and are listed in (Table 1). The values of D10, D50 and D90 in PCS were 14.00, 32.68 and 50.39, respectively. These values were similar to the correspondence values of formulation A and B. Therefore, in case of agitation granulation using PCS as an excipient, it was considered that HPC did not function as a binder. On the other hand, granules were formed when using dextrin, maltitol, and xylitol. From these results, low molecular weight binders such as oligosaccharides (dextrin) and sugar alcohols (xylitol, maltitol) were found to be useful for the preparation of granules containing PCS. Carr’s fluidity index of all of the prepared granules was more than 80, and the fluidity of each prepared granule was considered good and similar (Table 2). Good fluidity of granules can be attributed to the property of PCS as a fluidizing agent.
Pronounced sticking is the most problematic obstacle in the preparation of IBU tablets. In addition, the sticking becomes worse over the runtime of tableting because of the temperaturedependent mechanism of IBU. For a quantitative evaluation of sticking, measurement of surface roughness [47], scraper pressure [5], and ejection force have been reported [48]. In this study, we used two methods to determine the sticking of IBU tablets. After 1h of continuous tableting, the first method was the visual inspection of all tablets, and the second method was the examination of photographs of the upper punches with a digital camera.
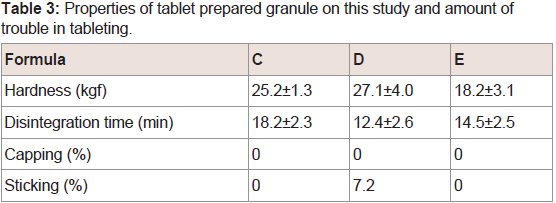
The state of the upper punch surface after tableting granules containing different binder is shown in (Figure 3). When the granules contained xylitol, a layer of powder was observed near the center of the punch. However, this layer of powder was not observed when the granules contained dextrin or maltitol. From the visual inspection, no defects were observed on the tablets after tableting with granules containing dextrin and maltitol. However, with xylitol, sticking was observed in 7.2% of tablets (Table 3). When the concentration of xylitol was increased by two-fold, sticking was not observed (datanot shown).In this study, we did not investigate the changes of the petal-like structure of PCS with the wet granulation method. But, it was possible to prepare tablets with high hardness (more than approximately 18 kgf) in low pressures (500 kg). This phenomenon suggested that an excellent formability of PCS cause by its petallike structure was not influenced through agitation granulation. Therefore, it was considered that the petal-like structure of PCS was not influenced with the wet granulation method.
Figure 3: Photographs of the upper punch surfaces after tableting using a)Dextrin, b) Xylitol, and c) Maltitol.
Dissolution test
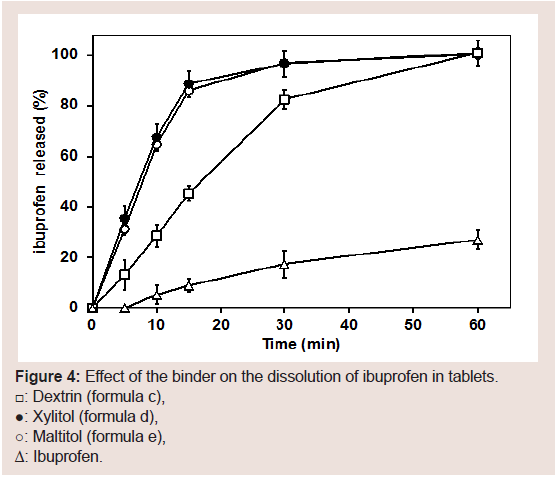
PCS has been used to improve drug dispersion and dissolution of tablets [40-43,49]. Our results of the dissolution study from the IBU tablets are shown in Figure 4. The raw material of IBU did not elute into the water for the most part, and the dissolution of IBU from the tablets and the PMs of tablet formulation containing PCS were good. Dissolution from the tablet prepared with dextrin was the lowest among the binders used in this study. These results suggested that the adhesion force of dextrin may be stronger than that of the other binders. Based on these results, PCS appears to be activated by water and functions to improve the dissolution of IBU in water. A process that uses high-speed agitation granulation to produce granules is crucial since IBU in the tablets eluted faster than from a bulk.
Figure 4: Effect of the binder on the dissolution of ibuprofen in tablets.☐; Dextrin (formula c),●; Xylitol (formula d),○; Maltitol (formula e),Δ: Ibuprofen.
Evaluation of IBU and binders in the granules
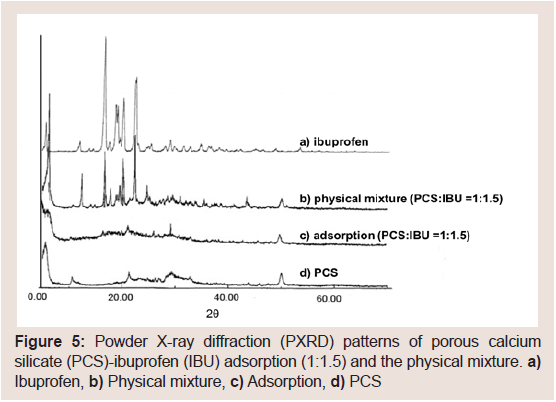
Figure 5 shows the PXRD patterns from the ASD and the PM of Formula C in (Table 1). X-ray diffraction peaks due to IBU crystals in the ASD were not observed. However, X-ray diffraction peaks in the PM were observed.
Figure 5: Powder X-ray diffraction (PXRD) patterns of porous calcium silicate (PCS)-ibuprofen (IBU) adsorption (1:1.5) and the physical mixture. a) Ibuprofen, b) Physical mixture, c) Adsorption, d) PCS
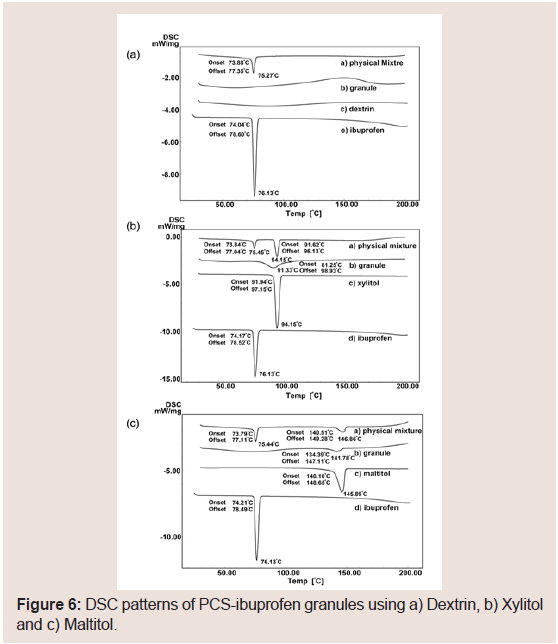
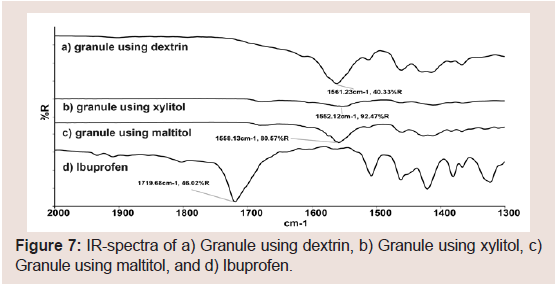
Figure 6shows the DSC thermograms of PCS-IBU granules with Dextrin (a), Xylitol (b), and Maltitol (c), respectively. IBU exhibited a melting endotherm at 76.13 °C. The melting endotherm of IBU was not observed in any PCS-IBU granule. From the results of PXRD and DSC, IBU may exist in an amorphous state in the granules prepared with PCS. Figure 7 shows the FT-IR spectra of obtained granules in this study. The acid C=O stretching at 1720 cm−1 of IBU in the granules shifts to near 1550 cm−1 on each granule, and the C=O stretching vibration of IBU does not shift, and similar phenomenon has been reported by Guo et al. [45]. Accordingly, state of IBU in the granules via the wet granulation method were considered the ionic state (a salt formation between the COO− groups of IBU and the Ca2+ ions of PCS) similar to ASD. From these results, quick dissolution of IBU from tablets in this study may have been caused by both of amorphization due to solid dispersion and ionization due to the interaction of IBU with calcium on PCS.
Figure 7: IR-spectra of a) Granule using dextrin, b) Granule using xylitol, c) Granule using maltitol, and d) Ibuprofen.
On the other hand, the effect of PCS on the melting endotherm of the binder was different. The melting endotherm of dextrin was not observed (Figure 6), because dextrin is a high molecular weight compound (average M.W. =26000). In the samples of xylitol and maltitol (Figure 6), the melting endotherms of the compounds in the PMs were the same as the pure compounds. However, the melting endotherms of xylitol and maltitol in the granules shifted to lower temperatures with broadening. These results suggest that the interaction may be a hydrogen bond that exists between PCS and the binders. To further determine this, FT-NIR measurements were carried out.
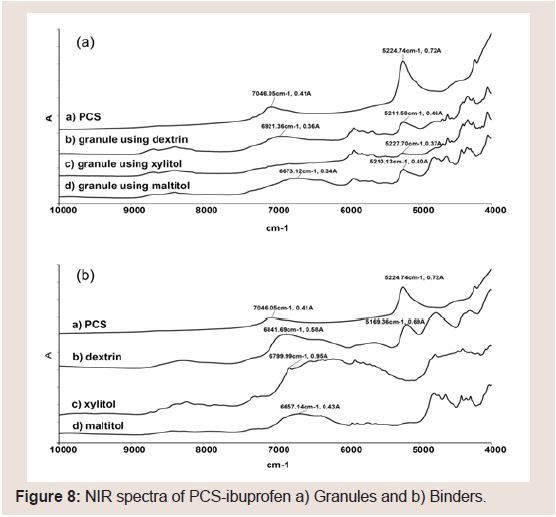
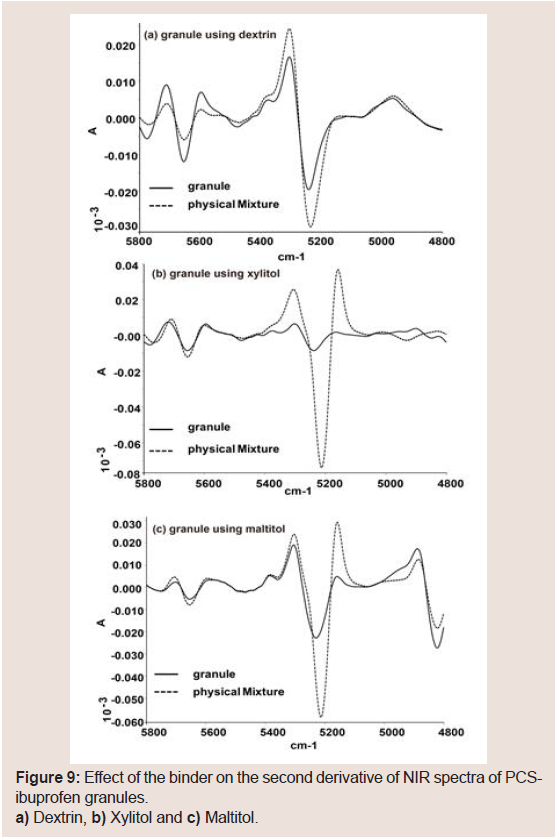
NIR spectra were measured to investigate potential interaction between PCS and the binders. The presence of a hydrogen bond-based interaction was confirmed using NIR spectroscopic investigations. Figure 8 shows the influence of the binder on the spectral data of PCS. The spectrum of pure PCS displayed a characteristic peak at 5225 cm−1 (H2O hydrogen bonded with -Si-OH) (Figure 8). Dextrin also displayed a peak at 5169 cm−1, but other samples did not display this peak. The dextrin peak was considered in that H2O hydrogen bonded with the hydroxy groups of the glucose unit and was assigned accordingly. NIR spectra of PCS-IBU granules with dextrin, xylitol, and maltitol are shown in Figure 8. The peak at about 5220 cm−1 was also observed in the raw dextrin samples. These results suggested that the peak at about 5200 cm−1 in the granule samples using dextrin may result from the -OH in dextrin. To remove the baseline shifting and improve peak resolution, a second derivative of the NIR spectra was obtained. Although the peak intensity at 5220 cm−1 decreased with the granule in the granules with dextrin compared with the PM Figure 9, the peak forms were almost the same between the granules and the PM. However, the peak intensity at 5220 cm−1 decreased in the granules with xylitol and maltitol Figure 9, particularly with xylitol. From these results, the intensity of the hydrogen bond between PCS and the binder may be xylitol>maltitol>dextrin.
Figure 9: Effect of the binder on the second derivative of NIR spectra of PCSibuprofen granules.a) Dextrin, b) Xylitol and c) Maltitol.
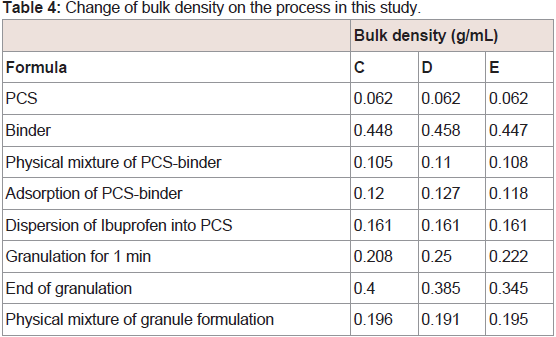
PCS possesses many interparticle (12 µm) and intraparticle (0.15 µm) pores on its surface [40]. Binders with a large intensity of hydrogen bonds may be able to distribute into the pores of PCS to a greater extent. In addition, it is considered that the distributed portion in the PCS pore depends on the molecular weight of the binder. From these considerations, xylitol (M.W.=152.15) may distribute into the deep portion of the PCS pore, while dextrin (average M.W.=26000) likely distributes on the surface portion of the PCS pore, while maltitol (M.W.=344.31) likely distributes in the middle portion of xylitol and dextrin. To investigate the main distributed portion of the binder in the PCS pore, the bulk density of the adsorption and granule sample was measured. If the bulk density (g/mL) of the sample (A) was larger than that of the sample (B), the sample (A) likely distributes on the surface portion of the PCS pore compared with the sample (B). The value of the bulk density of PCS is 0.062. The bulk densities of the other samples are listed in (Table 4). The values of dextrin granules, xylitol granules, and maltitol granules (same weight was PCS) were found to be 0.400, 0.345, and 0.385, respectively. The bulk density of the adsorption and the granules was dextrin>maltitol>xylitol. The IBU contents in dextrin, maltitol, xylitol, and IBU adsorption samples were the same. These results strongly support that xylitol mainly distributes to the deep portion of the PCS pores, with dextrin mainly distributed to the surface portion of the PCS pores, and maltitol distributed to the middle portion of xylitol and dextrin. From these results, the distributed portion of the binder in the PCS pore may affect the sticking property.
Conclusion
Solid dispersion granules were prepared with water-based solid dispersion using PCS property and wet granulation methods. The granules were prepared with dextrin, maltitol, and xylitol as binders, but HPC was not able to be used as a binder. Sticking was not observed with preparations containing dextrin and maltitol, but sticking was observed with xylitol. All prepared tablets demonstrated quick disintegration and dissolution. From DSC and PXRD studies, IBU may exist in an amorphous form in the ASD and the granules. From FT-IR studies, IBU may interact with PCS through a salt formation between the COO− groups of IBU and the Ca2+ ions of PCS. Therefore, quick dissolution of IBU from tablets in this study may have been caused by both of amorphization due to solid dispersion and ionization due to the interaction of IBU with calcium on PCS. Furthermore, from the results of DSC, NIR, and the bulk density of the binders, the main distributed portion of the specific binder in the PCS pores may differ among dextrin, maltitol, and xylitol, and this distribution in the PCS pore may affect the sticking property.
References
- Marshall K, Rudnic EM (1990) Tablet dosage forms. In: Banker GS, Rhodes CT, et al. (Eds), Modern Pharmaceutics 2ndedn, New York, USA, pp. 355-425.
- Danjo K, Kojima S, Chen CY, Sunada H, Otsuka A (1997) Efffect of water content on sticking during compression. Chem Pharm Bull 45: 706-709.
- Zuurman K, Maarschalk KV, Bolhuis GK (1999) Effect of magnesium stearate on bonding and porosity expansion of tablets produced from materials with different consolidation properties. Int J Pharm 179: 107-115.
- Kakimi K, Niwa T, Danjo K (2010) Influence of compression pressure and velocity on tablet sticking. Chem Pharm Bull (Tokyo) 58: 1565-1568..
- Danjo K, Kamiya K, Otsuka A (1993) Effect of temperature on the sticking of low melting point materials. Chem Pharm Bull 41: 1423-1427.
- Roberts M, Ford JL, MacLeod GS, Fell JT, Smith GW, et al. (2004) Effect of lubricant type and concentration on the punch tip adherence of model ibuprofen formulations. J Pharm Pharmacol 56: 299-305.
- Roberts M, Ford JL, MacLeod GS, Fell JT, Smith GW, et al. (2003) Effects of surface roughness and chrome plating of punch tips on the sticking tendencies of model ibuprofen formulations. J Pharm Pharmacol 55:1223-1228. .
- Saniocki I, Sakmann A, Leopold CS (2012) Direct compression of ibuprofen-containing powder blends: influence of the ibuprofen grade on the flow and compaction properties of an ibuprofen tablet formulation. Pharm Ind 74: 1842-1852.
- Rasenack N, Muller BW (2002) Properties of ibuprofen crystallized under various conditions: a comparative study. Drug Dev Ind Pharm 28: 1077-1089.
- Aoki S, Danjo K (1998) Absorption of ibuprofen coated by anhydrous Silicic acid. Chem Pharm Bull (Tokyo) 46: 1914-1917.
- Chen L, Dang Q, Liu C, Chen J, Song L, et al. (2012) Improved dissolution and anti-inflammatory effect of ibuprofen by solid dispersion. Front Med 6: 195-203.
- Bolhuis GK, Armstrong NA (2006) Excipients for direct compaction--an update. Pham Dev Technol 11: 111-124.
- Rasenack N, Muller BW (2002) Crystal habit and tableting behavior. Int J Pharm 244: 45-57.
- Rasenack N, Muller BW (2002) Ibuprofen crystals with optimized properties. Int J Pharm 245: 9-24.
- Bushra R, Shoaib M, Aslam N, Hashmat D, Ur-Rehman M (2008) Formulation development and optimization of ibuprofen tables by direct compression method. Pak J Pharm Sci 21: 113-120..
- Jivraj M, Martini LG, Thomson CM (2000) an overview of the different excipients useful for the direct compression of tablets. Pharm Sci Technol Today 3: 58-63.
- Jbilou M, Ettabia A, Guyot-Hermann AM, Guyot JC (1999) Ibuprofen agglomerates preparation by phase separation. Drug Dev Ind Pharm 25: 297-305.
- Zhou Q, Shi L, Marinaro W, Lu Q, Sun CC (2013) Improving manufacturability of an ibuprofen powder blend by surface coating with silica nanoparticles. Powder Technol 249: 290-296.
- Qu L, Zhou QT, Gengenbach T, Denman JA, Stewart PJ, et al. (2015) Investigation of the potential for direct compaction of a fine ibuprofen powder dry-coated with magnesium stearate. Drug Dev Ind Pharm 41: 825-837.
- Kocbek P, Baumgartner S, Kristl J (2006) Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int J Pharm 312: 179-186.
- Timpe C, Forschung L (2007) Strategies for formulation development of poorly water soluble candidates- A recent perspective. Am Pharm Rev 10: 104-109.
- Mann AK, Schenck L, Koynov A, Rumondor AC, Jin X, et al. (2018) Producing amorphous solid dispersions via co-precipitation and spray drying: Impact to physicochemical and biopharmaceutical properties. J Pharm Sci 107: 183-191.
- Kinoshita M, Baba K, Nagayasu A, Yamabe K, Shimooka T, et al. (2002) Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J Pharm Sci 91: 362-370.
- Hwang I, Kang CY, Park JB (2017) Advances in hot-melt extrusion technology toward pharmaceutical objectives. J Pharm Invest 47: 123-132.
- Shah S, Maddineni S, Lu J, Repka MA (2013) Melt extrusion with poorly soluble drugs. Int J Pharm 453: 233-252..
- Moneghini M, Kikic I, Voinovich D, Perissutti B, Filipovic-Grcic J (2001) Processing of carbamazepine-PEG 4000 solid dispersions with supercritical carbon dioxide: preparation, characterization, and in vitro dissolution. Int J Pharm 222: 129-138.
- Betageri GV, Makarla KR (1995) Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm 126: 155-160.
- Maniruzzaman M, Rana MM, Boateng JS, Mitchell JC, Douroumis D (2013) Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev Ind Pharm 39: 218-227.
- Pasquali I, Bettini R, Giordano F (2008) Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals. Adv Drug Deliv Rev 60: 399-410.
- Hu J, Johnston KP, Williams RO 3rd (2004) Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev Ind Pharm 30: 233-245.
- Leuner C, Dressman J (2000) Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 50: 47-60.
- Yamashita K, Nakate T, Okamoto K, Ohike A, Tokunaga Y, et al. (2003) Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm 267: 79-91.
- Knopp MM, Olesen NE, Holm P, Langguth P, Holm R, et al. (2015) Influence of polymer molecular weight on drug-polymer solubility: a comparison between experimentally determined solubility in PVP and prediction derived from solubility in monomer. J Pharm Sci 104: 2905-2912..
- Singh D, Pathak K (2013) Hydrogen bond replacement-unearthing a novel molecular mechanism of surface solid dispersion for enhanced solubility of a drug for veterinary use. Int J Pharm 441: 99-110..
- Planinsek O, Kovacic B, Vrecer F (2011) Carvedilol dissolution improvement by preparation of solid dispersions with porous silica. Int J Pharm 406: 41-48.
- Hanada M, Jermain SV, Williams RO 3rd (2018) Enhanced dissolution of a porous carrier-containing ternary amorphous solid dispersion system prepared by a hot melt method. J Pharm Sci 107: 362-371.
- Xia Y, Yuan M, Deng Y, Ke X, Ci T (2017) Different effects of silica added internal or external on in vitro dissolution of indomethacin hot-melt extrudates. Int J Pharm 534: 272-278.
- Ozeki T, Takashima Y, Nakano T, Yuasa H, Kataoka M, et al. (2011) Preparation of spray-dried microparticles using Gelucire 44/14 and porous calcium silicate or spherical microcrystalline cellulose to enhance transport of water-insoluble pranlukast hemihudrate across Caco-2 monolayers. Adv Powder Technol 22: 623-628.
- Kinoshita M, Baba K, Nagayasu A, Yamabe K, Shimooka T, et al. (2002) Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J Pharm Sci 91: 362-370.
- Yuasa H, Takashima Y, Kanaya Y (1996) Studies on the development of intragastric floating and sustained release preparation. I. Applicaation of calcium silicate as a floating carrier. Chem Pharm Bull 44: 1361-1366..
- Sharma S, Sher P, Badve S, Pawar AP (2005) Adsorption of meloxicam on porous calcium silicate: characterization and tablet formulation. AAPS Pharm Sci Tech 6: E618-E625.
- Fujimoto Y, Hirai N, Takatani-Nakase T, Takahashi K (2016) Preparation and evaluation of solid dispersion tablets by a simple and manufacturable wet granulation method using porous calcium silicate. Chem Pharm Bull (Tokyo) 64: 311-318.
- Fujimoto Y, Hirai N, Takatani-Nakase T, Takahashi K (2016) Novel tablet formulation of amorphous indomethacin using wet granulation with a high-speed mixer granulator combined with porous calcium silicate. J Drug Deliv Sci Technol 33: 51-57.
- Carr RL (1965) Evaluating flow properties of solids. Chem Eng J 72: 163-168.
- Guo X, Wu J, Yiu YM, Hu Y, Zhu YJ, et al. (2013) Drug-nanocarrier interaction--tracking the local structure of calcium silicate upon ibuprofen loading with X-ray absorption near edge structure (XANES). Phys Chem Chem Phys 15: 15033-15040..
- Hirai N, Ishikawa K, Takahashi K (2006) Improvement of the agitation granulation method to prepare granules containing a high content of a very hygroscopic drug. J Pharm Pharamcol 58: 1437-1441.
- Toyoshima K, Yasumura M, Ohnishi N, Ueda Y (1988) Quantitative evaluation of tablet sticking by surface roughness measurement. Int J Pharm 46: 211-215.
- Chambin O, Jannin V (2005) Interest of multifunctional lipid excipients: case of Gelucire 44/14. Drug Dev Ind Pharm 31: 527-534.
- Yuasa H, Asahi D, Takashima Y, Kanaya Y, Shinozuka K (1994) Application of calcium silicate for medicinal preparation. I. Solid preparation adsorbing an oily medicine to calcium silicate. Chem Pharm Bull (Tokyo) 42: 2327-2331.