Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
Preparation and Evaluation of Controlled-Release Solid Dispersion Granules Containing a Poorly Water-Soluble Drug, Hydrated Silicon Dioxide, and Polyvinylpyrrolidone
Uegaki Y, Hirai N, Takatani-Nakase T and Takahashi K*
Department of Pharmaceutics, Mukogawa Women’s University,
Hyogo, Japan
*Address for Correspondence: Koichi Takahashi, School of Pharmaceutical Sciences, Mukogawa
Women’s University, 11-68 Koshien, Kyuban-cho, Nishinomiya,
Hyogo 663-8179, Japan, Tel: +81 798 45 9943, Fax: +81 798 45 9943,
E-mail: koichi@mukogawa-u.ac.jp
Submission: 24 December 2018;
Accepted: 29 January 2019;
Published: 31 January 2019
Copyright: © 2019 Uegaki Y, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
The study aim was to develop controlled-release, solid dispersion
granules containing a poorly water-soluble drug, Hydrated Silicon
Dioxide (HSD), and Polyvinylpyrrolidone (PVP), and to elucidate the
mechanism underlyingsustained release from the soliddispersion
granules. To achieve this purpose, we used the wet granulation method
to prepare the first-release granules containing a poorly water-soluble
drug and HSD. Then, the effect of PVP on the dissolution of the poorly
water-soluble drug was estimated. Initially, the selection of a binder
and contentsof drug and binder were investigated to determine the
optimum formulation fora rapidly dissolving granule with HSD. Firstrelease
granules containing Nifedipine (NIF) as a poorly water-soluble
drug, erythritol as a binder, and HSD were developed. Differential
scanning calorimetry confirmed reduced NIF crystallinity in the granules.
To investigate the first-release granules’ applicabilityto other drugs, six
poorly water-soluble drugs (griseofulvin, indomethacin, ibuprofen,
carbamazepine, progesterone, and phenytoin) were prepared.
Rapid dissolutionof all tested drugs from the granule with the same NIF
formulation was observed. These findings suggest that HSD is useful for
improving dissolution ratesof poorly water-soluble drugs insoliddispersion
granules. Next, we investigated PVP’s effect on the dissolution of drug
from the first-release granules. The effects of PVP on sustained release
from the granules containing the seven drugs weredivided into three
types: Type 1 was no effect (rapid dissolution), type 2 was a middle
effect, and type 3 was a strong effect (sustained release). To elucidate
the mechanism underlying sustained release from the solid dispersion
granules, the intermolecular interactions between the drugs and HSD
or PVP were investigated by Fourier transform infrared spectroscopy.
The results suggested that the balance between the interaction of a
drug and HSD and the interaction of a drug and PVP is important for
sustained release of the drug.
Keywords
Poorly water-soluble drug; Controlled release; Hydrated
silicon dioxide; Polyvinylpyrrolidone K-90; Solid dispersion
Introduction
Oral drug administration is preferred because of its
convenience, good patient compliance, and low production costs.
Biopharmaceutical Classification System (BCS) class II compounds
exhibit low solubility and high permeability, which results in poor
bioavailability after oral administration [1]. Thus, improving these
drugs’ water solubility is one of pharmaceutical scientists’ current
strategies. To date, several approaches have been developed to
overcome this problem, including amorphous solid dispersions
[2], co-crystallization [3], and salt formation [4]. Particularly, an
amorphous solid dispersion is a system in which a poorly soluble drug is dispersed throughout a water-soluble carrier. Polyethylene
glycols, Hydroxypropyl Methylcellulose (HPMC), Poloxamer, and
Polyvinylpyrrolidone (PVP) are widely used solid dispersion carriers
because of their strong hydrophilic properties and ability to form
molecular adducts with many compounds [5,6].
In addition, inorganic materials, such as silica gel and calcium
silicate, improve the solubility of poorly water-soluble drugs [7-11].
Porous Calcium Silicate (PCS) and magnesium aluminosilicate
(Neusilin®) have huge surface areas and are used as solid dispersion
carriers to improve the dissolution of poorly water-soluble drugs
[10-12]. We reported the development of solid dispersion tablets
via a simple and easily manufactured wet granulation method using
PCS [13,14]. These materials contain silanol groups and metal
ions. The interaction between the drug and silanol groups or metal
ions likely affects the stability of the amorphous state and drug
dissolution rates [15,16]. Moreover, we have reported a hydrogen
bond between Nifedipine (NIF) and PCS as well as salt formation
between indomethacin and PCS [17]. Silicon dioxide (Carplex®,
Sylisia®, and Aerosil®) contains silanol groups but no metal ions.
Therefore, the effect of hydrogen bonds on the amorphous state and
drug dissolution rate may be investigated. The effect of silicon dioxide
on drug dissolution in the powder has been reported [18]. However,
there are few reports on the effect of silicon dioxide in the granules
and tablets.
Controlled-release, solid dispersion formulations should be
prepared for BCS class II drugs with short half-lives [19]. For this
purpose, different polymers, both hydrophobic (e.g., ethyl cellulose
and Eudragit) and hydrophilic (e.g., hydroxypropyl cellulose, HPMC,
and methylcellulose), have been used [20-23]. In the polymer matrix
system, a drug is homogeneously distributed throughout a matrix,
and drug release is controlled by water-swell able or hydrophobic
polymeric excipients. Moreover, silica is used to prepare controlled release
solid dispersion formulations along with a hydrophobic
polymer to control drug release [24]. Recently, we reported sustained
release from NIF-PCS granules containing a hydrophilic polymer, PVP, and rapid release from indomethacin-PCS granules containing
PVP [17]. We suggested that hydrogen bonds among the drug, PCS,
and PVP contribute to this sustained release. However, it is not clear
whether PVP affects the release of a poorly water-soluble drug from
the solid dispersion formulation containing other silicates, and the
underlying mechanism of the sustained release also remains unclear.
The purpose of this study was to develop controlled-release solid
dispersion granules, containing poorly water-soluble drugs, Hydrated
Silicon Dioxide (HSD), and PVP, and to elucidate the mechanism
underlying sustained release from the solid dispersion granules. We
prepared first-release granules containing a poorly water-soluble
drug and HSD. Then, the effect of PVP on the dissolution of several
poorly water-soluble drugs was estimated. Because we hypothesized
that one of the mechanisms underlying the sustained release may
involve interaction between the drug and polymer, we also measured
the interactions between the drugs and HSD or PVP by Fourier
transform infrared (FT-IR) spectroscopy.
Materials and Methods
Materials:
NIF and indomethacin were purchased from Permachem Asia
Ltd. (Tokyo, Japan) and KONGO CHEMICAL Co., Ltd. (Toyama,
Japan), respectively. Griseofulvin, ibuprofen, and carbamazepine
were obtained from Wako Pure Chemical Industries Co., Ltd. (Osaka,
Japan). Phenytoin and progesterone were purchased from Tokyo
Chemical Industry Co., Ltd. (Tokyo, Japan) and Nacalai Tesque Ltd.
(Kyoto, Japan), respectively. HSD (Carplex® #80) was obtained from
DSL Japan Co., Ltd. (Tokyo, Japan). PVP K-90 was purchased from
Nacalai Tesque Ltd. Mannitol, trehalose, fructose, sorbitol, erythritol,
and xylitol, which were used as binders, were obtained from Nacalai
Tesque Ltd., Asahi Kasei Corp. (Tokyo, Japan), Kato Kagaku Co., Ltd.
(Aichi, Japan), Towa Chemical Industry Co., Ltd. (Osaka, Japan),
Mitsubishi-Chemical Foods Corp. (Tokyo, Japan), and B Food
Science Co., Ltd. (Tokyo, Japan), respectively. All other chemicals
were of reagent grade and used without further purification.Preparation of solid dispersion granules:
To prepare the rapidly dissoluble, solid dispersion granules, the
drug was dissolved in an appropriate amount of ethanol (50 g) by
heating at 60 °C. The solution was added to HSD (40 g) and mixed
for 15 min by using a high-speed agitation granulator (High-Speed
Mixer, EARTHTECHNICA Co., Ltd., Tokyo, Japan) at 250 rpm with
an agitator and 2500 rpm with a chopper. After drying at 70 °C for
12 h, the powdery binder and water (50 g) were added to this dried
mixture to prepare the granules by using the granulator at 250 rpm
with an agitator and 2500 rpm with a chopper. For the experiment to
estimate the effect of PVP on dissolution from the solid dispersion
granule, PVP (10 g) was added to the binder suspension. The
granulation end point was visually determined. The granules were
dried at 70 °C for 12 h and then pulverized in a speed mill (Okada
Seiko Co., Ltd., Tokyo, Japan). Granules ranging in size from 500 to
850 μm were used in this study.Preparation of the adsorption solid dispersion (ASD) and physical mixture (PM):
To prepare an ASD of drug and HSD, an appropriate amount of
drug was dissolved in ethanol (10 g) by heating at 60 °C. Then, this
solution was added to HSD (8 g) and mixed for 15 min by using a
rotation mixer. The mixture was dried at 70 °C for 12 h. The PM was
prepared by mixing an appropriate amount of drug and HSD (8 g).Drug-release experiments:
Dissolution tests were performed, according to the JP17 paddle
method, using Riken’s Dissolution Tester (Miyamoto Riken Ind
Co., Ltd., Osaka, Japan). Granules containing 10 mg of drug were
added to the dissolution medium (900 mL of purified water) at 37
°C±0.5 °C, and the paddle was rotated at 50 or 75 rpm. The amounts
of dissolved NIF, indomethacin, carbamazepine, progesterone,
griseofulvin, and phenytoin were analyzed by using an Ultraviolet
(UV) spectrophotometer (UV-1200; Shimadzu Corp., Kyoto, Japan)
at 350, 320, 285, 241, 292, and 258 nm, respectively. The amount of
dissolved ibuprofen and granules’ contents were analyzed by highperformance
liquid chromatography using a Shimadzu LC-10ADvp
pump (Shimadzu Corp.), a Shimadzu SPD-20A detector (Shimadzu Corp.) set at 230 nm, and an L-column ODS (4.6 mm x 150 mm, 5
μm; Chemicals Evaluation and Research Institute, Japan). The mobile
phase consisted of acetonitrile-10 mM KH2PO4 in a 55:45 (v/v) ratio,
and the flow rate was 1.0 mL/min. All analyses were performed at
40 °C. Three granule samples were tested in each batch, and the
mean values were calculated. The drug content in the granules was
estimated using a UV spectrophotometer. An adequate amount of
granules equivalent to 10 mg drug was accurately weighed, dissolved,
and suitably diluted in methanol and measured by using a UV
spectrophotometer.Differential scanning calorimetry (DSC):
DSC analyses were performed using an automatic thermal
analyzer (DSC-60 Plus; Shimadzu Corp.) and an indium standard for
temperature calibrations. Holed aluminum pans were employed in
the experiments for all samples, and an empty pan, prepared in the
same way, was used as a reference. Samples (1-10 mg) were sealed
in the aluminum pans, and heating curves were recorded by using a
constant heating rate of 5°C/min from 30 °C to 350 °C.Fourier transforms infrared spectroscopy (FT-IR):
IR spectra of powder samples were obtained using a
spectrophotometer (IRAffinity-1; Shimadzu Corp.) and the potassium
bromide (KBr) pellet method. KBr disks were prepared by mixing
several milligrams of the sample with KBr and compacting. The scan
range was 400-4000 cm-1.Results and Discussion
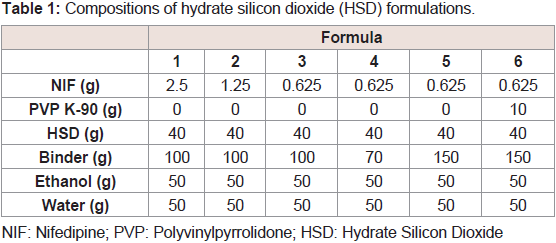
Preparation and optimization of rapidly dissolving solid dispersion granules containing NIF and HSD:
To obtain the optimum formulation of rapidly dissolving granules
with HSD, the selection of the binder and contents of the drug and
binder were investigated.NIF was used as the model of a poorly
water-soluble drug. Four sugar alcohols (erythritol, D-mannitol,
xylitol, and sorbitol) and two sugars (trehalose and fructose) were
selected for preparing granules on the basis of formula 1 using the
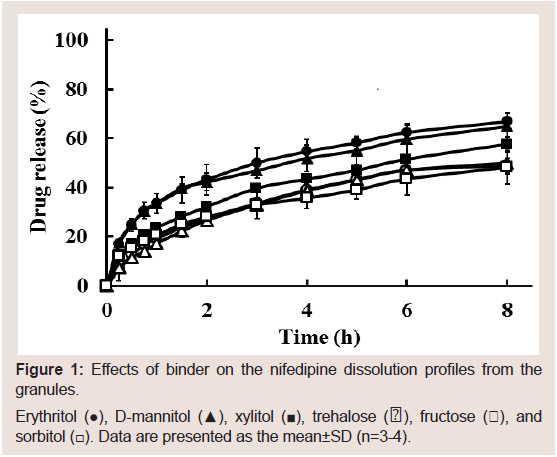
wet granulation method Table 1. The dissolution rates of granules
prepared with D-mannitol and erythritol were higher than those of
granules prepared with other sugar alcohols. Granules prepared with
sorbitol had the lowest dissolution rate (Figure 1). Sugimoto et al.
have suggested that the dissolution rate of oral tablets was in the order
of erythritol>mannitol> xylitol> glucose>sorbitol [25]. These results indicate that erythritol is the best substance for penetration of water
into granules or tablets.
Figure 1: Effects of binder on the nifedipine dissolution profiles from the
granules.
Erythritol (●), D-mannitol (▲), xylitol (■), trehalose (〇), fructose (△), and
sorbitol (□). Data are presented as the mean±SD (n=3-4).
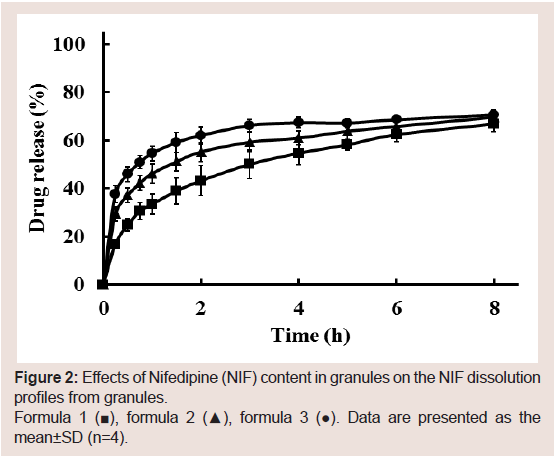
Furthermore, the effect of NIF content in granules on the NIF
dissolution rate was investigated (formulas 1-3). The granules’
dissolution rates improved with decreasing NIF content (Figure 2).
Since the NIF content used in the dissolution test was constant (10
mg), a decrease in the granules’ NIF content was proportional to a
required increase in granules used in the dissolution test. There are
two possible reasons for this effect: 1) increased NIF dissolution rates
from the granules and 2) a portion of the NIF in the granules existed
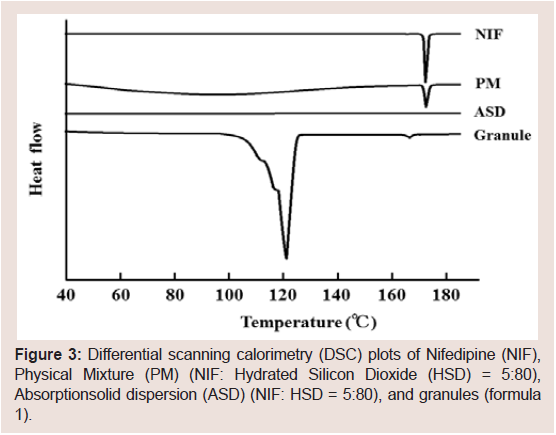
in a crystalline state rather than anamorphous state. To confirm this, DSC was performed. Figure 3 shows the DSC thermograms of
the ASD, PM, and granules (formula 1). NIF exhibited a melting
endotherm at 172 °C. A DSC peak was not observed in the ASD and
granules because of the NIF crystals but it was observed in the PM.
These results suggested that the main reason for improved granules’
dissolution rates as the NIF content decreased was the increased NIF
dissolution rate from the granules.
Figure 2: Effects of Nifedipine (NIF) content in granules on the NIF dissolution
profiles from granules.
Formula 1 (■), formula 2 (▲), formula 3 (●). Data are presented as the
mean±SD (n=4).
Figure 3: Differential scanning calorimetry (DSC) plots of Nifedipine (NIF),
Physical Mixture (PM) (NIF: Hydrated Silicon Dioxide (HSD) = 5:80),
Absorptionsolid dispersion (ASD) (NIF: HSD = 5:80), and granules (formula
1).
When more granules were used in the dissolution test, many of
them were not stirred well and precipitated. Therefore, the paddle
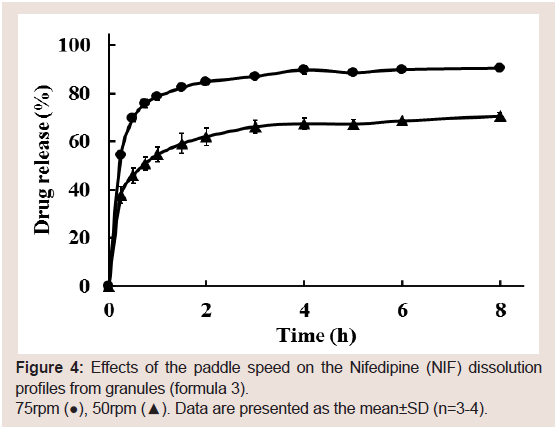
speed’s effect on NIF dissolution was investigated (formula 3). The
NIF dissolution rate increased with an increase in paddle speed from
50 to 75 rpm (Figure 4). On the basis of these results, the paddle speed
was set to 75 rpm in the following dissolution study.
Figure 4: Effects of the paddle speed on the Nifedipine (NIF) dissolution
profiles from granules (formula 3).
75rpm (●), 50rpm (▲). Data are presented as the mean±SD (n=3-4).
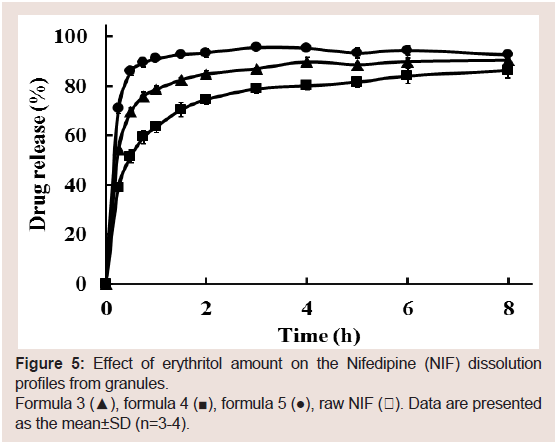
To further optimize the NIF granules’ solid dispersion formulation, the effect of binder content (erythritol) on the NIF
dissolution rate from the granules was investigated (formulas 3-5).
The dissolution rate increased with increasing erythritol content
(Figure 5). The increased dissolution rate was because of the granules’
faster disintegration time as the amount of erythritol was increased.
On the basis of these results, formula 5 was selected as the best
formulation in this study.
Figure 5: Effect of erythritol amount on the Nifedipine (NIF) dissolution
profiles from granules.
Formula 3 (▲), formula 4 (■), formula 5 (●), raw NIF (〇). Data are presented
as the mean±SD (n=3-4).
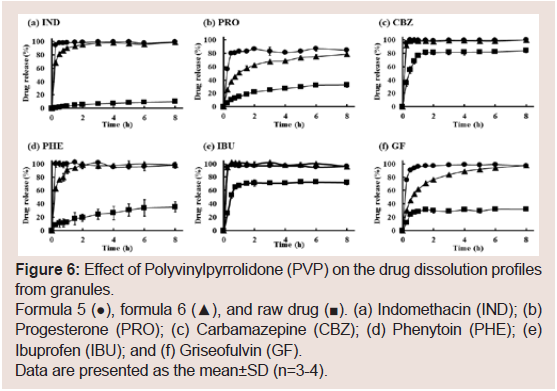
Development of HSD granule to other drugs:
To investigate the possibility of applying the preparation
method of the rapidly dissolving granules to other drugs (formula
5), six poorly water-soluble drugs [Griseofulvin (GF), Indomethacin
(IND), Ibuprofen (IBU), Carbamazepine (CBZ), Progesterone
(PRO), and Phenytoin (PHE)] were selected (Figure 6). shows these
drugs’ dissolution rates from the granules as well as the raw drugs.
All drugs used in this study showed rapid dissolution. However, the dissolutions of NIF, GF, PRO, and IND within shorter times (from
15 min to 60 min) appeared to be different from those of other drugs.
This difference maybe because of the interaction between the drug
and HSD.
Figure 6: Effect of Polyvinylpyrrolidone (PVP) on the drug dissolution profiles
from granules.
Formula 5 (●), formula 6 (▲), and raw drug (■). (a) Indomethacin (IND); (b)
Progesterone (PRO); (c) Carbamazepine (CBZ); (d) Phenytoin (PHE); (e)
Ibuprofen (IBU); and (f) Griseofulvin (GF).
Data are presented as the mean±SD (n=3-4).
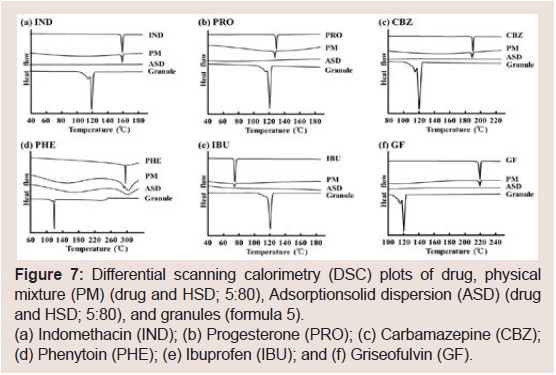
DSC thermograms were examined to investigate the drugs’
crystallinity in HSD formulations (Figure 7). Samples of ASD or PM
at a 5:80 drug to HSD ratio and granules (formula 5) were used in this
study. The drugs’ endothermic peaks were observed for the raw drugs
and the PM samples. However, endothermic peaks were not observed
in the ASD samples. From these results, all six drugs may exist in the
amorphous state in the ASD samples. Because the amount of drug in
formula 5 was lower than that in the ASD sample, shown in (Figure 7), we considered that all six drugs in the HSD granules existed in the
amorphous state.
Figure 7: Differential scanning calorimetry (DSC) plots of drug, physical
mixture (PM) (drug and HSD; 5:80), Adsorptionsolid dispersion (ASD) (drug
and HSD; 5:80), and granules (formula 5).
(a) Indomethacin (IND); (b) Progesterone (PRO); (c) Carbamazepine (CBZ);
(d) Phenytoin (PHE); (e) Ibuprofen (IBU); and (f) Griseofulvin (GF).
Effect of PVP on the dissolution of drugs from HSD granules:
Recently, we studied the effect of a hydrophilic polymer, PVP,
on the dissolution of a poorly water-soluble drug (NIF) from PCS
granules (rapidly dissolving granules) and reported the sustained
release from PCS granules [17]. In the present study, we prepared
rapid dissolution granules containing a poorly water-soluble drug,
binder, and HSD. Granules containing PVP were prepared (formula
6) to investigate PVP’s effect on the dissolution from the rapid
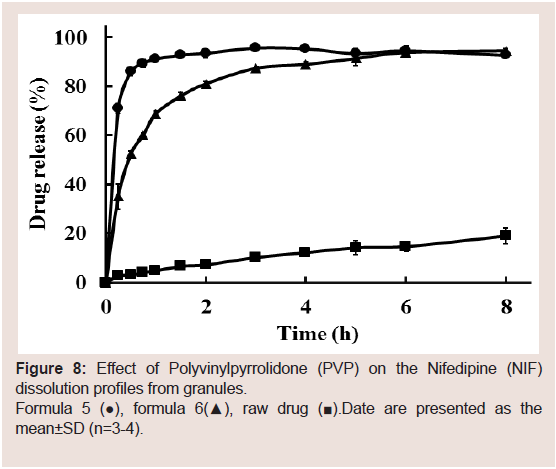
dissolution granules. (Figure 8) shows PVP’s effect on the dissolution
of NIF from the granules. Sustained NIF release was observed with
addition of PVP. PVP’s effects on the dissolution of the other six drugs
from the granules were also investigated (Figure 6). In CBZ and IBU,
no effect of PVP on dissolution was observed. On the other hand, in
PRO and GF, sustained release of the drugs was observed from the
granules containing PVP. In NIF, IND, and PHE, sustained release of drugs was observed at early dissolution times. From the effect of PVP
to sustained release, we divided these drugs into three types: Type
1 (IBU and CBZ) was no effect, type 2 (NIF, IND, and PHE) was a
moderate effect and type 3 (PRO and GF) was a strong effect.
Figure 8: Effect of Polyvinylpyrrolidone (PVP) on the Nifedipine (NIF)
dissolution profiles from granules.
Formula 5 (●), formula 6(▲), raw drug (■).Date are presented as the
mean±SD (n=3-4).
Interaction of drug with PVP or HSD:
We previously suggested that sustained NIF release was due to
hydrogen bonding among NIF, PVP, and PCS [17]. Therefore, the
interactions among a drug, PVP, and HSD may affect the drug release
from the granule. To further investigate PVP’s effect on dissolution,
the intermolecular interactions between a drug and HSD or PVP were
measured by FT-IR. To measure the interaction of drug and polymer
in more detail, we prepared ASD and PM at drug: HSD or drug: PVP
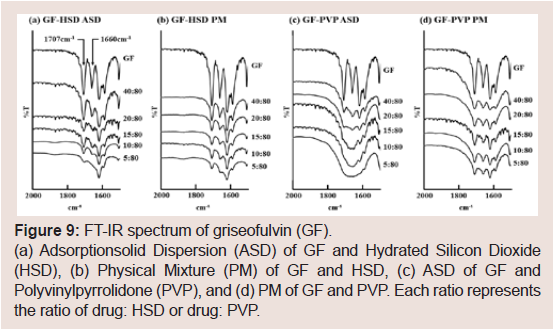
ratios ranging from 5:80 to 40:80.The FT-IR spectra of GF are shown in (Figure 9). The spectrum of
raw GF shows characteristic peaks at 1660 cm-1 and 1707 cm-1 (C=O
stretching) [26]. These peaks were observed in all PM samples (Figure
9b and d), whereas the 1660 cm-1 peak in the ASD samples was not
observed at GF: HSD ratios of 20:80 and GF: PVP of 15:80 but was
observed at GF: HSD ratios of 40:80 and GF: PVP of 20:80 (Figure
9a and c).
Figure 9: FT-IR spectrum of griseofulvin (GF).
(a) Adsorptionsolid Dispersion (ASD) of GF and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of GF and HSD, (c) ASD of GF and
Polyvinylpyrrolidone (PVP), and (d) PM of GF and PVP. Each ratio represents
the ratio of drug: HSD or drug: PVP.
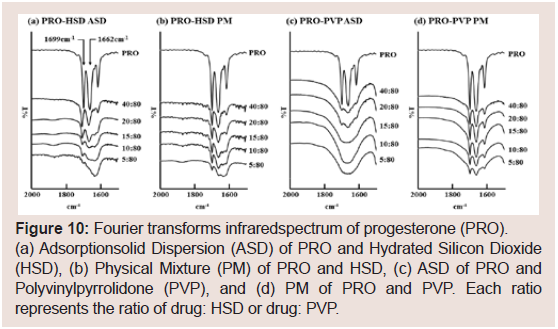
The PRO’s FT-IR spectra are shown in Figure 10. The raw PRO
spectrum shows characteristic peaks at 1662 cm-1 and 1699 cm-1 (C=O
stretching) [26], which were observed in all PM samples (Figure 10b and 10d). Additionally, the ASD samples of HSD showed a peak of
1662cm-1 at PRO: HSD ratios above 20:80 (Figure 10a). In the ASD
samples with PVP (Figure 10c), the characteristic two peaks were
observed at PRO: PVP ratios above 20:80.
Figure 10: Fourier transforms infrared spectrum of progesterone (PRO).
(a) Adsorptionsolid Dispersion (ASD) of PRO and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of PRO and HSD, (c) ASD of PRO and
Polyvinylpyrrolidone (PVP), and (d) PM of PRO and PVP. Each ratio
represents the ratio of drug: HSD or drug: PVP.
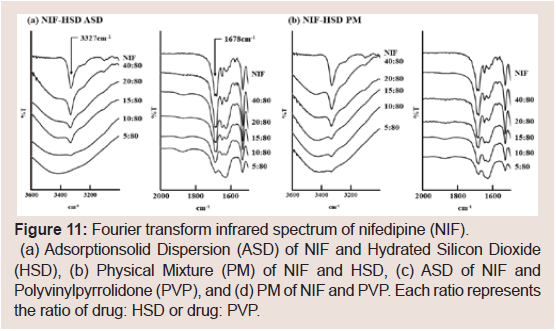
The FT-IR spectra of NIF are shown in (Figure 11). The spectrum
of raw NIF shows the characteristic peaks at 1678 (C=O stretching)
and 3327 cm-1 (secondary -NH) [27]. These peaks were observed in
the PM samples of all ratios of NIF: HSD and PVP (Figure 11b and
d). In the ASD samples, the peaks were observed at ratios of NIF:
HSD=15:80 and NIF: PVP=40:80 (Figure 11a and 11c).
Figure 11: Fourier transform infrared spectrum of nifedipine (NIF).
(a) Adsorptionsolid Dispersion (ASD) of NIF and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of NIF and HSD, (c) ASD of NIF and
Polyvinylpyrrolidone (PVP), and (d) PM of NIF and PVP. Each ratio represents
the ratio of drug: HSD or drug: PVP.
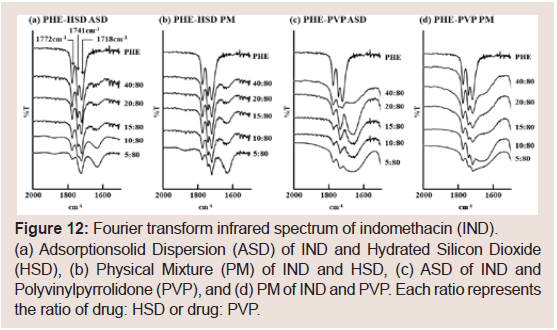
The PHE’s FT-IR spectra are shown in (Figure 12). The spectrum
of raw PHE shows characteristic peaks at 1718, 1772cm-1 (C=O
stretching), and 1741 cm-1 (bending -NH) [28]. These peaks were
observed in all PM samples (Figure 12b and 12d). In the sample at PHE:
HSD=5:80, the peak at 1741 cm-1 was not observed but was observed
at PHE: HSD=10:80 (Figure 12a). In the ASD samples with PVP
(Figure 12c), the characteristic three peaks were observed at ratios
above PHE: PVP=40:80.
Figure 12: Fourier transform infrared spectrum of indomethacin (IND).
(a) Adsorptionsolid Dispersion (ASD) of IND and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of IND and HSD, (c) ASD of IND and
Polyvinylpyrrolidone (PVP), and (d) PM of IND and PVP. Each ratio represents
the ratio of drug: HSD or drug: PVP.
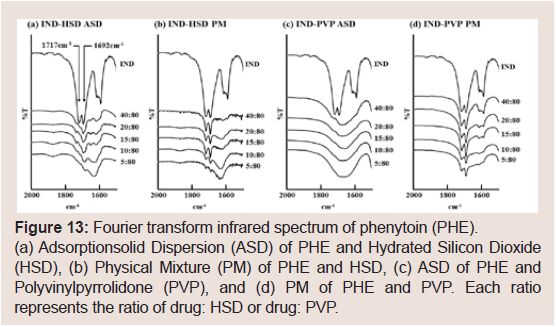
The IND’s FT-IR spectra are shown in Figure 13. The spectrum
of raw IND shows characteristic peaks at 1717 cm-1 (acid C=O
stretching) and 1692 cm-1 (benzoyl C=O stretching) [29]. These peaks
were observed in all PM samples (Figure 13b and 13d) and in the sample
of ASD at IND: HSD=15:80 (Figure 13a). In the ASD samples with
PVP (Figure 13c), the characteristic two peaks were observed at ratios
above IND: PVP=40:80.
Figure 13: Fourier transform infrared spectrum of phenytoin (PHE).
(a) Adsorptionsolid Dispersion (ASD) of PHE and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of PHE and HSD, (c) ASD of PHE and
Polyvinylpyrrolidone (PVP), and (d) PM of PHE and PVP. Each ratio
represents the ratio of drug: HSD or drug: PVP.
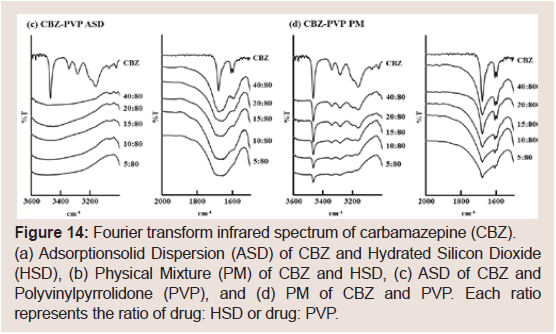
Figure 14 shows the CBZ’s FT-IR spectra. The raw CBZ spectrum
shows characteristic peaks at 1595 and 1605 cm-1 (bending -NH),
1677cm-1 (C=O stretching), and 3466 and 3161 cm-1 (secondary -NH)
[30]. These peaks were observed in all PM samples (Figure 14b and 14d).
In the sample of ASD at CBZ: HSD=10:80, these peaks were observed
(Figure 14a). On the other hand, these peaks were not observed in the
sample of ASD with PVP (Figure 14c).
Figure 14: Fourier transform infrared spectrum of carbamazepine (CBZ).
(a) Adsorptionsolid Dispersion (ASD) of CBZ and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of CBZ and HSD, (c) ASD of CBZ and
Polyvinylpyrrolidone (PVP), and (d) PM of CBZ and PVP. Each ratio
represents the ratio of drug: HSD or drug: PVP.
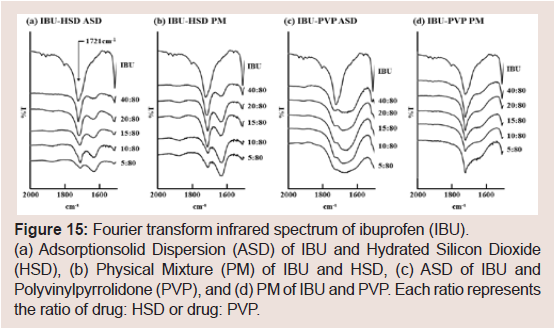
The IBU’s FT-IR spectra are shown in Figure 15. The raw IBU
spectrum shows the characteristic peak at 1721cm-1 (acid C=O
stretching) [31]. In HSD, this peak was observed in all samples of
both PM and ASD (Figure 15a and 15b). On the other hand, in PVP,
the peak was not observed in any of the ASD samples (Figure 15c).
Figure 15: Fourier transform infrared spectrum of ibuprofen (IBU).
(a) Adsorptionsolid Dispersion (ASD) of IBU and Hydrated Silicon Dioxide
(HSD), (b) Physical Mixture (PM) of IBU and HSD, (c) ASD of IBU and
Polyvinylpyrrolidone (PVP), and (d) PM of IBU and PVP. Each ratio represents
the ratio of drug: HSD or drug: PVP.
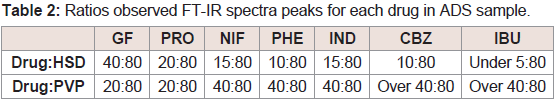
Table 2 shows the ratios at which the FT-IR spectra peaks for each
drug were observed in the ASD sample of drug and HSD or PVP.
FT-IR spectroscopy can provide information on molecular states,
including the components, their crystal forms, and the formation
of intermolecular interactions. Peak shifts and broadening in the
spectra reflect the interactions in solid dispersions and co-amorphous
systems. In this study, the drug peaks were not observed at ratios
smaller than the drug ratios listed in Table 2. These phenomena may
be explained by the polymers’ dilution effect. However, the peaks of
drug were observed in the PM samples at all ratios. The broadening
(disappearance) of drug peaks in the ASD samples reflects the
interaction in the solid dispersion between the drug and polymer.
The drug peaks were observed at higher ratios of drug than the ratios
listed in Table 2. This finding suggests that drugs in the ASD samples
present more than drugs able to interact with a polymer. Drugs that
are unable to interact with a polymer may be in a crystalline form
in the system. Therefore, the observation of peaks at a higher drug
ratio might be explained by the stronger interaction of the drug with
a polymer than that of other drugs or other polymers.
In type 1 (CBZ and IBU), the drug peaks were observed in the
samples at all ratios with PVP and at low ratios with HSD. These
results suggest that type 1 drugs in the granules strongly interact with
PVP and weakly interact with HSD. This indicates the release rate
from the granules containing HSD and PVP may be controlled only
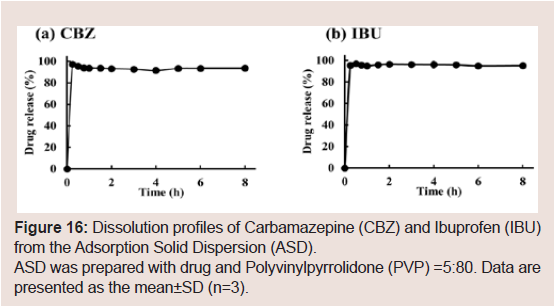
by PVP. Figure 16 shows the dissolution curves from ASD with PVP
and CBZ or IBU. Similar release curves are shown in Figure 16c,e
and Figure 16. The interaction of type 2 with HSD may be stronger
than that of type 3, and the interaction of type 2with PVP may be
weaker than that of type 3. The interaction of type 2 with PVP may
be stronger than that with HSD, and the interaction of type 3 with
HSD may be nearly the same strength as that with PVP. From these
considerations, the balance between the interaction of drug and HSD
and the interaction of drug and PVP appear to be important for the
drugs’ sustained release.
Conclusion
Solid dispersion formulations of NIF with HSD were prepared by
using the wet granulation method and evaluated. The formulations
exhibited much higher dissolution rates than the NIF powder. NIF
was present in an amorphous state in the granules. The formulation
can also be applied to other poorly water-soluble drugs. Solid
dispersion granules with HSD may be useful for improving the
dissolution rates of poorly water-soluble drugs. Furthermore, granules containing one of six poorly water-soluble drugs, HSD, and
PVP were prepared to evaluate PVP’s effect on the drugs’ sustained
release. The effects of PVP were divided into three types: Type 1 was
no effect, type 2 was a moderate effect, and type 3 was a strong effect.
To elucidate the mechanism underlying sustained release from the
solid dispersion granules, the intermolecular interaction between a
drug and HSD or PVP was investigated by FT-IR. The study results
suggest that the balance between the interaction of a drug and HSD
and the interaction of a drug and PVP is important for the sustained
release of drugs.