Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
Inhibitory Effect of 5,7-Dimethoxyfl avone On Rosuvastatin Uptake From The Apical Membrane Of Caco-2 Cells
Kimura O1, Ohta C2, Koga N2, Kato Y3, Haraguchi K4 and Endo T1*
1School of Pharmaceutical Sciences, Health Sciences University
of Hokkaido, 1757 Kanazawa, Ishikari-Tobetsu, Hokkaido 061-
0293, Japan
2Faculty of Nutritional Sciences, Nakamura Gakuen University,
Johnan-Ku, Fukuoka 814-0198, Japan
3Kagawa School of Pharmaceutical Sciences, Tokushima Bunri
University, Sanuki, Kagawa 769-2193, Japan
4Department of Pharmaceutical Sciences, Daiichi University of
Pharmacy, Minami-Ku, Fukuoka 815-8511, Japan
Address for Correspondence:
Endo T, School of Pharmaceutical Sciences, Health Sciences
University of Hokkaido, 1757 Kanazawa, Ishikari-Tobetsu,
Hokkaido 061-0293, Japan; Tel: +81 090 7655 5403; E-mail:
endotty531115@gmail.com
Submission: 11 October 2022
Accepted: 12 November 2022
Published: 15 November 2022
Copyright: © 2022 Endo T, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
5,7-Dimethoxyfl avone (5,7-DMF) is a natural polymethoxyfl avone,
and acts as an inhibitor of ABC efflux transporters (BCRP, MRP2 and/
or P-gp), and rosuvastatin is taken up via OATP2B1 and secreted by
BCRP and MRP2. In this study, we investigated the effect of 5,7-DMF
on the transport of rosuvastatin through the apical membrane and the
accumulation in Caco-2 cells. Furthermore, we investigated whether
the rosuvastatin accumulation is mediated by monocarboxylate
transporter 1 (MCT1), in addition to OATP2B1. Coincubation with 5,7-
DMF significantly increased the cellular accumulation of rosuvastatin
from the apical membranes of Caco-2 cells cultured on the plastic
dish. Coincubation with Ko-143 (a BCRP inhibitor) or MK-571 (an
MRP inhibitor) significantly increased the rosuvastatin accumulation,
whereas coincubation with verapamil (a P-gp inhibitor) did not.
Coincubation with benzoic acid or pravastatin, which are substrates
of both OATP2B1 and MCT1, signifi cantly decreased the rosuvastatin
accumulation, whereas coincubation with estron-3-sulfate or
sulfobromophthalein, which are substrates of both OATP2B1 and ABC
efflux transporter, did not decrease and increased the rosuvastatin
accumulation, respectively. On the other hand, the transcellular
transport of rosuvastatin from basolateral to apical side (B-to-A
transport) was markedly higher than that from apical to basolateral
side (A-to-B transport). Coincubation with 5,7-DMF from the apical
side significantly increased the A-to-B transport and the accumulation
of rosuvastatin, whereas that from the basolateral side significantly
decreased the B-to-A transport of rosuvastatin with increases in the
accumulation. These results suggest that 5,7-DMF may increase the
rosuvastatin accumulation as a result of the inhibition of rosuvastatin
efflux mediated by BCRP and MRP2, and the rosuvastatin transport
through the apical membrane may be mediated by not only OATB2B1
but also MCT1.
Keywords
5,7-Dimethoxyflavone; Rosuvastatin; P-glycoprotein (P-gp);
Breast cancer resistance protein (BCRP); Multidrug resistance protein
2 (MRP2); Organic anion transporting polypeptide 2B1 (OATP2B1);
Monocarboxylate transporter 1 (MCT 1); Pravastatin; Caco-2 cells
Introduction
Most membrane transporters belong to two super-families, ABC
(ATP-binding cassette) and SLC (solute-linked carrier). Three major
efflux transporters of the ABC family are P-glycoprotein (P-gp),
breast cancer resistance protein (BCRP), and multidrug resistance
protein 2 (MRP2). These efflux transporters, localized on the brush
border membrane of the enterocytes, can limit drug absorption by
pumping substrate into the luminal side against a concentration
gradient, using ATP as an energy source [1,2].
Organic anion transporting polypeptides (OATPs) belong to
the SLC super-family and mediate the cellular uptake of various
endogenous and exogenous amphiphilic organic compounds in the
brain, liver, lung, kidney, testes, intestine, etc. OATP1B1, OATP1B3,
and OATP2B1 are OATPs expressed in the liver, and mediated the
uptake of various compounds in the liver [3]. In contrast, OATP2B1 is the main OATP expressed in the human intestine and Caco-2 cells,
and the amount of OATP2B1 expression is markedly higher than that
of other OATP isomers such as OATP1A2, OATP3A1, OATP4A1,
OATP1B1 and OATP1B3 [4-6]. The physiological function of
OATP2B1 is distinctly Na+-independent and pH-gradient dependent
with a relatively narrow substrate specificity compared to other
OATPs [7]. OATP2B1 could play an important role in oral absorption
of various compounds such as sulfobromophthalein (BSP), statins,
fexofenadine, glibenclamide, etc. in addition to the physiological
sulfate-conjugated steroids and taurocholate [1-3,8].
There is some overlap in apparent substrate specificity between
OATP2B1 and monocarboxylate transporter 1 (MCT1). Compounds
that are reported to be substrates of both OATP2B1 and MCT1 include
nicotinate, benzoic acid, salicylic acid, pravastatin, atorvastatin, etc.,
and MCT1-mediated transport is enhanced by an increase of H+
concentration [1,7], as in the case of OATP2B1. Furthermore, OATPs
shares with some substrates of ABC efflux transporters [9]; BSP and
estron-3-sulfate (E3S), typical substrates of OATP2B1, are likely to
act as the substrates of MRP2 and BCRP [10,11], respectively.
Lipid-lowering drugs of 3-hydroxy-3-methylglutaryl-coenzyme
A (HMG-CoA) reductase inhibitors (statins), such as rosuvastatin,
pravastatin, atorvastatin, pitavastatin, fluvastatin, simvastatin and
lovastatin, are used in the treatment and prevention of atherosclerotic
disease [12,13]. Most statins are absorbed from the intestinal
lumen mainly by OATP2B1 [9,14-17], and secreted (pumped out)
by one or some of BCRP, MRP2 and P-gp [12,18-22]. The statins,
except for simvastatin and lovastatin, are monocarboxylic acids,
and the absorption of pravastatin and atorvastatin are reported to
be mediated by MCT1 [7], in addition to OATP2B1. However, the uptake of other statins having monocarboxylic acid by MCT1 has
not yet been investigated. Some statins are used as inhibitors of ABC
efflux transporter(s), because of their high affinity for ABC efflux
transporter(s) [8,11,23].
A methylated flavone, 5,7-dimethoxyflavone (5,7-DMF) is an
inhibiter of BCRP, MRP2, and/or P-gp [24-27]. As 5,7-DMF has high
oral absorption and bioavailability as compared to non-methylated
flavones, with low toxicity and little metabolism [28,29]. 5,7-DMF
is promising for use as a chemosensitizing agent for the BCRP and
MRP2-mediated anticancer drug resistance [25,26,30-32].
For instance, 5,7-DMF significantly increased the mitoxantrone
accumulation (a BCRP substrate) in BCRP-expressing MDCK cells
[32], and 5,7-DMF increased doxorubicin accumulation in A549
cells by the inhibition of MRP-mediated efflux [25]. 5,7-DMF is a
major constituent flavonoid of Kaempferia parviflora, and the dose
of K parviflora tincture ingested in herbal medicine (30 mL) is likely
to increase the intestinal absorption of drugs which is mediated by
MRP2 [25]. However, the mechanism of intestinal absorption of 5,7-
DMF has not yet been investigated, and only a few reports focused on
the intestinal interaction of 5.7-DMF and drug which is mediated by
MRP2, BCRP, and/or P-gp have been reported.
The human colorectal adenocarcinonoma cell line Caco-2 is
morphologically and functionally similar to human small intestinal
epithelial cells and is widely used to predict intestinal ‘‘in vivo’’
absorption in humans [33]. These cells spontaneously differentiate
in culture into polarized cell monolayers with many enterocyte-like
properties of transporting epithelia and also remain ABC and SLC
transporters. Caco-2 cells are an appropriate model to investigate
drug absorption as well as drug-drug and food-drug interactions
caused by uptake and efflux transporters [34].
As mentioned above, rosuvastatin and 5,7-DMF are reported to
be substrates (inhibitors) of some ABC efflux transporters, BCRP,
MRP2, and/or P-gp, and rosuvastatin is reported to be a substrate
of uptake transporter of OATP2B1. The aim of this study was to
investigate the effect of 5,7-DMF on the intestinal absorption of
rosuvastatin using Caco-2 cells. Furthermore, we investigated the
uptake and efflux of rosuvastatin using typical substrates (inhibitors)
of membrane transporters, in particular, whether rosuvastatin is
taken up by MCT1 or not.
Materials & Methods
Materials:
Rosuvastatin calcium and pravastatin sodium were obtained
from the Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
5,7-Dimethoxyfl avone (5,7-DMF), verapamil hydrochloride, benzoic
acid, cyclosporin A (ciclosporin A) and Dulbecco’s modified Eagle’s
medium (DMEM) were purchased from FUJIFILM Wako Pure
Chemical Industries, Ltd. (Osaka, Japan). MK-571, zosuquidar
hydrochloride, sulfobromophthalein (BSP), estron-3-sulfate (E3S),
and quercetin were purchased from Sigma-Aldrich (St. Louis, MO).
Ko-143 was purchased from Tocris Bioscience (Minneapolis, MN).
All other chemicals used were of the highest purity commercially
available. Rosuvastatin and some compounds were dissolved in
dimethyl sulfoxide (DMSO) and added to the incubation medium at
a final concentration of DMSO of 1% or lower.Cell culture:
As reported previously [35,36], Caco-2 cells between passages
55 and 75 were maintained in a culture medium that is DMEM
containing 10% fetal bovine serum (FBS), 1% nonessential amino
acid, streptomycin (100 μg/mL) and penicillin G (100 U/mL) at 37°C
in a humidified atmosphere of 5% CO2 in the air.Cellular accumulation of rosuvastatin:
Caco-2 cells were grown on 35-mm six-well culture dishes coated
with rat tail collagen type I (Corning Incorporated, Tewksbury, MA)
at a density of 5 × 104 cells/well in 1.5 mL of culture medium [35,36].
After seeding, confluent Caco-2 cell monolayers cultured for 14-16
days were used in the cellular accumulation study.The incubation medium used in this study was Hanks’ balanced
salt solution containing 10 mM MES at pH 6.0 or 10 mM HEPES
at pH 7.4. The culture medium was removed, and the cells were
preincubated at 37°C for 20 min in 1.5 mL of the incubation medium
at pH 7.4. After preincubation, the incubation medium was aspirated,
and the cells were incubated in the medium at pH 6.0 or 7.4 containing
rosuvastatin for the designated time at 37°C.
To investigate the mechanisms underlying the cellular
accumulation of rosuvastatin, the cells were coincubated with 10
μM rosuvastatin and several transporter inhibitors (substrates). To
investigate ATP-dependent rosuvastatin efflux, the cellular ATP was
depleted by the pretreatment with 10 mM NaN3 and 10 mM NaF at
37°C for 20 min before the incubation with 10 μM rosuvastatin [37].
Furthermore, to investigate the effects of low temperature on the
cellular accumulation of rosuvastatin, the cells were preincubated
at 4°C and pH 7.4 for 20 min, and then incubated with 10 μM
rosuvastatin at 4°C and pH 6.0 for 30 min.
After incubation with rosuvastatin, the cell surface was quickly
washed three times with an ice-cold incubation medium. The cells
were suspended in 1.0 mL of extraction solution (1 N H3PO4: methanol
= 1:1) for 60 min at room temperature, and the cells were scraped
off and collected using a cell scraper [35,36]. The cell suspension
was centrifuged at 13,000g for 10 min, and a 100-μL aliquot of the
supernatant was injected into the HPLC system.
Efflux of rosuvastatin:
The efflux experiment was performed as described previously
[35]. Caco-2 cells were incubated 10 μM rosuvastatin at pH 6.0 and
37°C for 30 min. After incubation, the cell surface was quickly washed
three times with an ice-cold incubation medium. The washed cells
were then incubated at 37°C and pH 6.0 for the designated time in
the incubation medium containing 100 μM 5,7-DMF or an inhibitor
of ABC efflux transporter (10 M Ko-143, 50 μM MK-571 or 100 μM
verapamil), or the washed cells were incubated in the inhibitor-free
medium at 4°C and pH 6.0. After the designated time, the rosuvastatin
remaining in the cells was measured as described above.Transcellular transport of rosuvastatin:
Caco-2 cells were seeded on 6-well polyethylene terephthalate
Falcon® cell culture inserts (0.4 μm pores, 4.2-cm2 growth area;
Corning Incorporated, Tewksbury, MA) at a density of 5 × 104
cells/well, and then cultured for 21-23 days in DMEM containing
10% FBS, streptomycin (100 μg/mL) and penicillin G (100 U/mL) [35]. The monolayers grown on confluency, with a transepithelial
electrical resistance of more than 350 Ω cm2, were used for the
transport experiment. The cells were preincubated at 37°C for 20
min in 1.5 and 2.5 mL of the incubation medium at pH 7.4 from the
apical and basolateral sides, respectively. After preincubation, the
cell monolayers were incubated with 10 μM rosuvastatin or 10 μM
rosuvastatin and 100 μM 5,7-DMF for the designated time at 37°C
either from the apical side of the monolayers at pH 6.0 (1.5 mL) or
from the basolateral side at pH 7.4 (2.5 mL). After the incubation
with rosuvastatin, the transcellular transport of rosuvastatin was
measured, and the cellular accumulation of rosuvastatin was also
measured after the suspension of cells in the extraction solution.To investigate the effects of several compounds on the transcellular
transport and the cellular accumulation of rosuvastatin from the
apical side, Caco-2 cells were coincubated with 10 μM rosuvastatin
and several transporter inhibitors (10, 100, and 200 μM 5,7-DMF, 100
μM verapamil, 10 μM Ko-143 and 50 μM MK-571).
Apparent permeability coefficient from apical to basolateral side
(Papp A-B) and from basolateral to apical side (Papp B-A) were calculated
using volume of the incubation medium (1.5 or 2.5 mL), filter surface
area (4.2 cm2), and initial concentration in rosuvastatin (10 μM) [19].
Determination of rosuvastatin and protein concentrations:
Determination of rosuvastatin was carried out using an HPLC
system consisting of a Shimadzu LC-20Avp pump and SPD-10A
UV detector (Kyoto, Japan) equipped with an Inertsil ODS-SP 5
μM column (4.6 mm i.d. × 250 mm; GL Sciences, Tokyo, Japan).
The mobile phase consisted of acetonitrile–0.1% phosphoric acid
(40 : 60, v/v), and the flow rate was 1.0 mL min-1. The wavelength
was 242 nm, and the column was kept at 40°C. The calibration curve
of rosuvastatin was linear over the range of 0.01-0.20 nmol/mL (r =
0.99). The protein concentration was determined using a Bio-rad dye
reagent (Richmond, CA) with bovine serum albumin as the standard.Statistical analyses:
Data were shown as the mean ± S.E. Data were analyzed by
Student’s t-test, and p value below 0.05 was regarded as statistically
significant.Results
Effects of extracellular pH and 5,7-DMF on the cellular accumulation of rosuvastatin:
accumulation of rosuvastatin
Caco-2 cells were incubated with 10 μM rosuvastatin at pH 6.0 or
7.4 for up to 120 min in the presence or absence of 100 μM 5,7-DMF
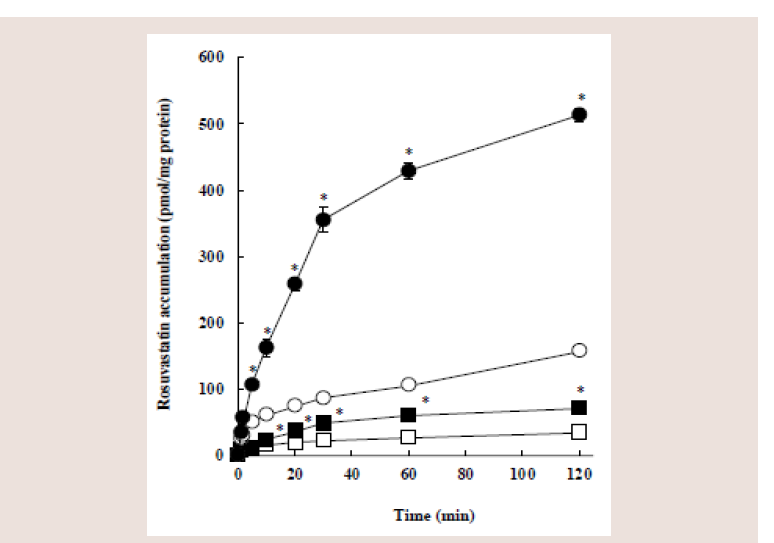
(Figure 1).
Figure 1: Eff ects of pH and 5,7-DMF on the cellular accumulation of
rosuvastatin in Caco-2 cells. Caco-2 cells were incubated with 10 μM
rosuvastatin for the indicated periods in the presence (closed symbols) or
absence (open symbols) of 100 μM 5,7-DMF at pH 6.0 (circles) or pH 7.4
(squares). Each value represents the mean ± S.E. of 4-6 determinations.
*Significantly different from the control (without 5,7-DMF).
The cellular accumulation of rosuvastatin after the incubation
with 10 μM rosuvastatin at pH 6.0 or 7.4 increased with time,
however, the accumulation at pH 6.0 was several-hold higher than
that at pH 7.4.
Coincubation with 5,7-DMF significantly increased the
rosuvastatin accumulation at pH 6.0 and 7.4, but this increase at pH
6.0 owing to 5.7-DMF was greater than that at pH 7.4: At 30, 60, and
120 min, the accumulation at pH 6.0 in the presence of 5.7-DMF was
3-5-hold higher than that in the absence of 5,7-DMF, whereas the
accumulation at pH 7.4 in the presence of 5,7-DMF was only 2-hold
higher than that in the absence of 5,7-DMF. Notably, the rosuvastatin accumulation at pH 6.0 in the presence of 5,7-DMF increased greatly
and almost linearly up to 30 min.
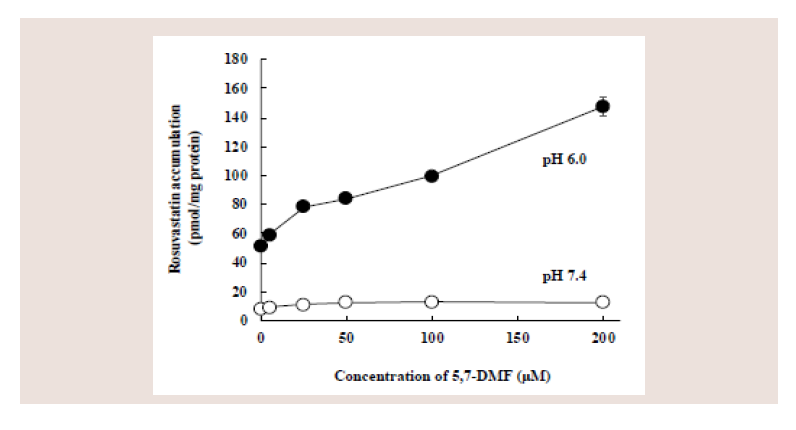
Figure 2 shows the concentration-dependent effect of 5,7-DMF
on the rosuvastatin accumulation. The rosuvastatin accumulation
at 5 min and pH 6.0 was apparently increased with the increasing
5,7-DMF concentration (5, 25, 50, 100, and 200 μM). In contrast,
the increasing concentration of 5,7-DMF did not increase the
rosuvastatin accumulation at pH 7.4.
Figure 2: Effect of 5,7-DMF on the cellular accumulation of rosuvastatin in
Caco-2 cells. Caco-2 cells were coincubated at pH 6.0 or 7.4 for 5 min with
10 μM rosuvastatin and different concentrations of 5,7-DMF. Each value
represents the mean ± S.E. of 4-6 determinations.
Effects of 5,7-DMF on the rosuvastatin efflux from Caco-2 cells:
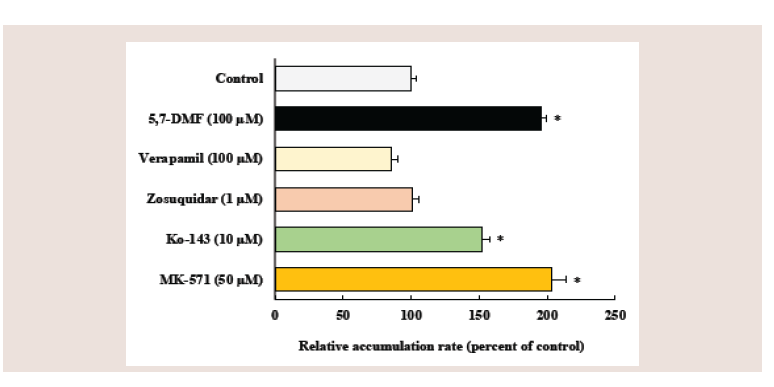
Figure 3 shows the effects of 5,7-DMF and ABC efflux inhibitors
on the rosuvastatin accumulation in Caco-2 cells. Coincubation
with 100 μM 5,7-DMF significantly increased the rosuvastatin
accumulation by 2.0-fold. Similarly, coincubation with 10 μM Ko-
143 (a BCRP inhibitor) or 50 μM MK-571 (an MRP2 inhibitor)
significantly increased the rosuvastatin accumulation by 1.5-hold and
2.1-hold, respectively. In contrast, coincubation with P-gp inhibitor,
1 μM zosuquidar or 100 μM verapamil, did not affect the rosuvastatin
accumulation, although these concentrations of P-gp inhibitors are
reported to inhibit the P-gp mediated efflux [38,39].
Figure 3: Effects of 5,7-DMF and inhibitors of ABC efflux transporters on
the cellular accumulation of rosuvastatin in Caco-2 cells. Caco-2 cells were
coincubated with 10 μM rosuvastatin and various compounds at pH 6.0
for 5 min. Each value represents the mean ± S.E. of 4-6 determinations.
*Significantly different from the control.
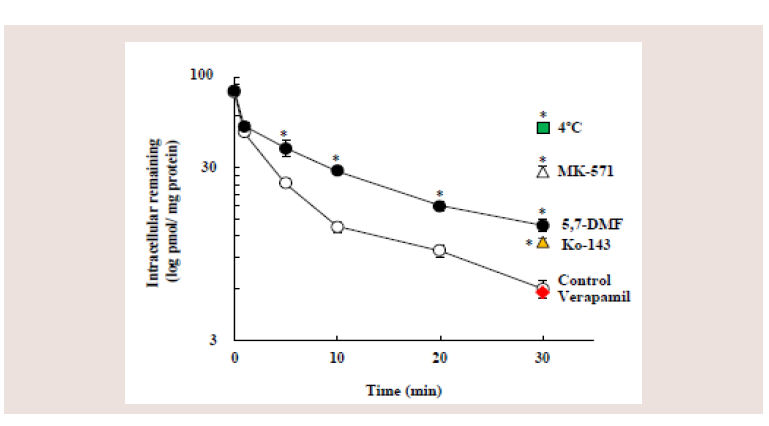
The amount of the rosuvastatin accumulation decreased rapidly
with time (control), showing a 93% decrease in the rosuvastatin
accumulation at 30 min. 5,7-DMF (100 μM) significantly inhibited
the rosuvastatin efflux at 5, 10, 20 and 30 min. At 30 min, 100 μM
5,7-DMF, 10 μM Ko-143 and 50 μM MK-571 significantly inhibited
the rosuvastatin efflux by 83, 86 and 65%, respectively. In contrast,
100 μM verapamil did not inhibit the rosuvastatin efflux at 30 min
(93%), whereas low temperature (4°C) markedly decreased the efflux
(38%) (Figure 4).
Figure 4: Effects of 5,7-DMF and inhibitors of ABC efflux transporters on the
efflux of rosuvastatin from Caco-2 cells. Caco-2 cells, incubated with 10 μM
rosuvastatin at 37ºC for 30min, were further incubated at 37ºC for designed
time with or without 100 μM 5,7-DMF at pH 6.0. Furthermore, Caco-2 cells,
incubated with rosuvastatin, were further incubated at 37ºC for 30 min with
MK-571, Ko-143 or verapamil, or further incubated at 4ºC for 30 min with no
chemicals. Each value represents the mean ± S.E. of 4-6 determinations.
*Significantly different from the control.
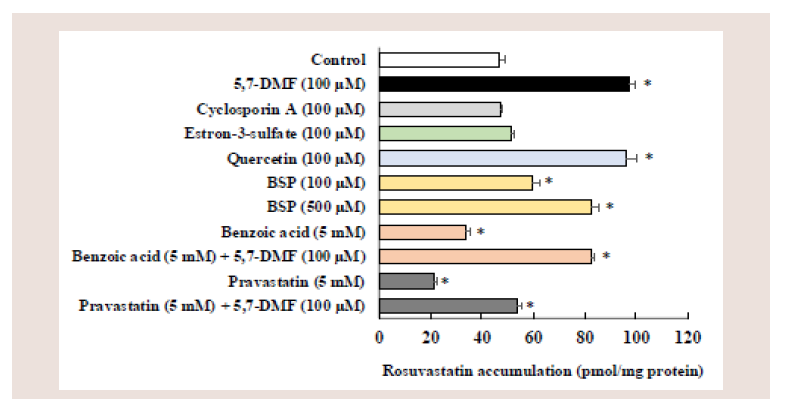
Comparison of rosuvastatin accumulation by various inhibitors:
We investigated typical inhibitors of transporters involved in the
rosuvastatin accumulation (Figure 5), some of which are known to act
as the ABC transporter inhibitors as mentioned in the Introduction.
Figure 5: Eff ects of various compounds on the cellular accumulation of
rosuvastatin in Caco-2 cells. Caco-2 cells were incubated with 10 μm
rosuvastatin with or without of various compounds at pH 6.0 for 5 min.
Each value represents the mean ± S.E. of 3-6 determinations. *Significantly
different from the control.
Coincubation with 100 μM cyclosporin A or 100 μM E3S did
not affect the rosuvastatin accumulation. In contrast, coincubation with 100 μM quercetin or 500 μM BSP significantly increased the
rosuvastatin accumulation by 2.0-fold and 1.8-fold, respectively,
as in the case of 5.7-DMF (2.0-fold), whereas coincubation with
a low concentration of 100 μM BSP marginally increased the
rosuvastatin accumulation by 1.3-fold. In contrast, coincubation
with 5 mM pravastatin or 5 mM benzoic acid signifi cantly decreased
the rosuvastatin accumulation by 55 and 29%, respectively, and the
decreased accumulation by pravastatin and benzoic acid was greatly
enhanced in the presence of 100 μM 5,7-DMF which significantly
exceeded the control level.
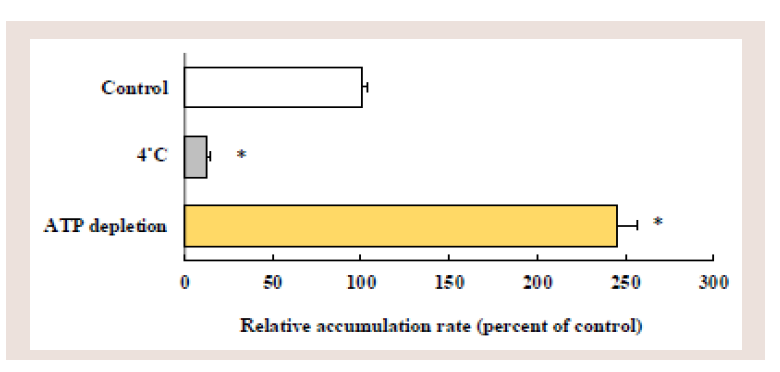
Effects of low temperature and ATP-depletion on rosuvastatin accumulation:
To confirm the involvement of uptake transporter(s) and
ATP (energy)-dependent efflux transporters in the rosuvastatin
accumulation, Caco-2 cells were incubated with rosuvastatin at 4°C,
or ATP-depleted Caco-2 cells were incubated with rosuvastatin at
37°C (Figure 6).
Figure 6: Effects of low temperature and ATP depletion on the cellular
accumulation of rosuvastatin in Caco-2 cells. Caco-2 cells were incubated
with 10 μM rosuvastatin at 37˚C or 4˚C, or ATP-depleted Caco-2 cells were
incubated with 10 μM rosuvastatin at 37˚C. Each value represents the mean
± S.E. of 4-6 determinations. *Significantly different from the control.
The rosuvastatin accumulation was drastically decreased by the
incubation at low temperature (4°C) by 87%. In contrast, the rosuvastatin
accumulation was greatly increased by ATP depletion (2.4-fold).
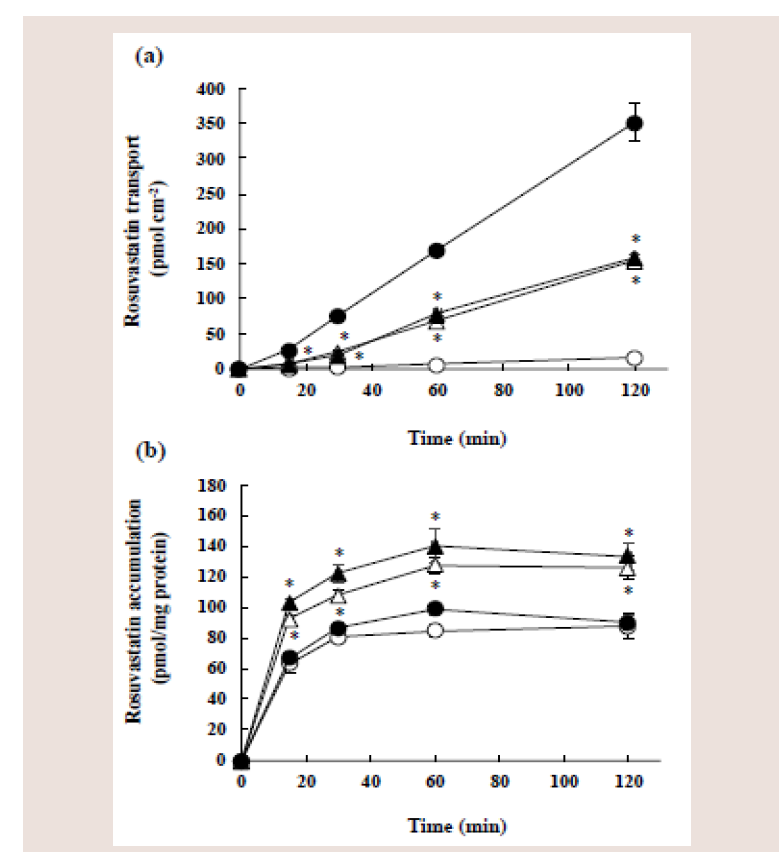
Effects of 5,7-DMF on the transcellular transport and accumulation of rosuvastatin after either apical or basolateral side of incubation:
Effects of 5,7-DMF and other efflux transporter inhibitors on the transcellular transport and the accumulation of rosuvastatin were
investigated using Caco-2 cell monolayers cultured on permeable
membranes. Caco-2 cell monolayers were incubated with 10 μM
rosuvastatin at 37°C for the designated time either from the apical
side at pH 6.0 or the basolateral side at pH 7.4.The rosuvastatin transport from the apical to the basolateral side
(A-to-B) and from the basolateral to apical side (B-to-A) increased
linearly with time (Figure 7a). However, the B-to-A transport at each
time was about 20-fold greater than the A-to-B transport: The Papp
A-B and Papp B-A were 0.24 (± 0.02) × 10-6 cm/s and 5.13 (± 0.94) × 10-6
cm/s, respectively.
Figure 7: Effect of 5,7-DMF on the transcellular transport (a) and cellular
accumulation (b) of rosuvastatin in Caco-2 cell monolayers. Caco-2 cell
monolayers were incubated with 10 μM rosuvastatin with (triangles) or without
(circles) 100 μM 5,7-DMF from either the apical side at pH 6.0 (open symbols)
or basolateral side at pH 7.4 (closed symbols). Each value represents the
mean ± S.E. of 4-6 determinations. *Significantly different from the control
(without 5,7-DMF).
Coincubation with 5,7-DMF significantly increased the A-to-B
transport of rosuvastatin, whereas it significantly decreased the
B-to-A transport: The Papp A-B and Papp B-A of rosuvastatin in the
presence of 5.7-DMF were 2.37 (± 0.25) × 10-6 cm/s and 2.48 ( ± 0.18)
× 10-6 cm/s, respectively. Thus, a great difference between Papp B-A and
Papp A-B in the absence of 5,7-DMF was diminished by the presence of
5,7-DMF.
On the other hand, the rosuvastatin accumulation at pH 6.0 and
7.4 in the presence of 5,7-DMF was significantly higher than that in
the absence of 5,7-DMF, and the difference in the accumulation due
to the difference in pH was not observed (Figure 7b). The rosuvastatin
accumulation at pH 6.0 and 7.4 after 60 min attained plateau levels,
irrespective of 5,7-DMF.
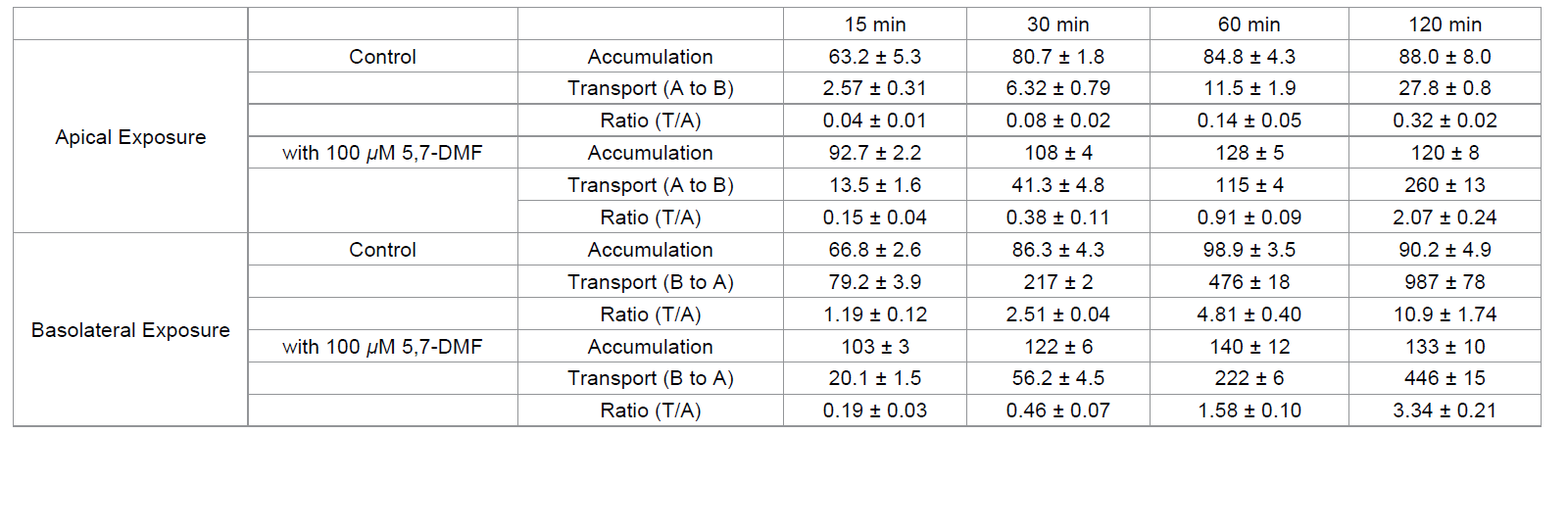
Table 1 shows the rosuvastatin accumulation in tissue (A), the
transcellular transport (T), and the ratio (T/A) in the experiment
shown in Figure 7a and Figure 7b. The ratios (T/A) incubated from the
apical side in the absence of 5,7-DMF increased gradually with time
(0.04-0.32), whereas the ratios in the reverse direction increased
sharply (1.19-10.9), in agreement with the preferential transport of
rosuvastatin from the basolateral side (Figure 7a). On the other hand,
the ratios from apical side exposure were increased by 5,7-DMF (0.15–
2.07), while the ratios from basolateral side exposure were decreased
(0.19-3.34). In the presence of 5,7-DMF, no particular differences
were found in the ratios between the exposure of rosuvastatin from
the apical and basolateral sides.
Table 1: Ratio of transcellular transport to accumulation of rosuvastatin in Caco-2 cells incubated with rosuvastatin in the presence or absence of 5,7-DMF from the
apical compartment or basolateral compartment.
Effects of efflux transporter inhibitors on transcellular transport and accumulation of rosuvastatin after apical of incubation:
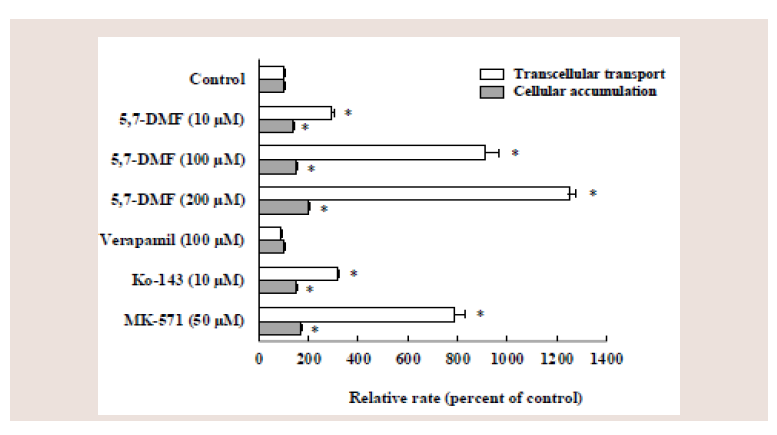
The eff ects of 5,7-DMF, Ko-143, MK-571 and verapamil on the
A-to-B transport and the accumulation of rosuvastatin at 60 min
were compared (Figure 8). The inhibitors and their concentrations
used in this experiment were the same as in the previous experiment
(Figure 3). The pH of the apical and basolateral medium was 6.0 and
7.4, respectively.
Figure 8: Effects of several compounds on the apical-to-basolateral transport
and cellular accumulation of rosuvastatin in Caco-2 cell monolayers. Caco-2
cell monolayers were coincubated for 60 min at pH 6.0 with 10 μM rosuvastatin
and various compounds from apical side. Each value represents the mean ±
S.E. of 4-6 determinations. *Significantly different from the control.
Coincubation with different concentrations of 5,7-DMF (10,
100 and 200 μM) significantly increased the rosuvastatin transport
by 2.9-, 9.1- and 13-fold, respectively, and significantly increased the
rosuvastatin accumulation by 1.4-, 1.5- and 2.0-fold, respectively.
Coincubation with 10 μM Ko-143 or 50 μM MK-571 significantly
increased the rosuvastatin transport by 3.2- and 7.9-fold, respectively,
and significantly increased the rosuvastatin accumulation by 1.5-
and 1.7-fold, respectively. In contrast, coincubation with 100 μM
verapamil did not affect the transport and the accumulation of
rosuvastatin.
Discussion
5,7-DMF significantly increased the rosuvastatin accumulation at
pH 6.0 (Figure 1), and the A-to-B transport and the accumulation of
rosuvastatin from the apical side (Figure 7a and b). Furthermore, 5,7-
DMF increased the accumulation and the transport of rosuvastatin
at pH 6.0 in a concentration-dependent manner (Figure 2 and 8).
As in the case of 5,7-DMF, Ko-143 (a BCRP inhibitor) and MK-
571 (an MRP2 inhibitor) significantly increased the transport and the accumulation of rosuvastatin at pH 6.0, whereas verapamil and
zosuquidar (P-gp inhibitors) did not (Figure 3 and 8). Th us, 5,7-DMF
appears to increase the rosuvastatin accumulation in Caco-2 cells at
pH 6.0 as a result of the inhibition of rosuvastatin efflux mediated by
BCRP and MRP2, but not by P-gp. The efflux study strengthens the
inhibitory effect of 5,7-DMF on the rosuvastatin efflux mediated by
BCRP and MRP2 (Figure 4).
The rosuvastatin accumulation at pH 6.0 in the presence of 5,7-
DMF increased greatly and almost linearly up to 30 min (Figure 1).
A similar increase was reported in talinolol accumulation in cultured
cells which was mediated by Oatp and P-gp [40]. The great and
linear increase of the rosuvastatin accumulation at pH 6.0 is likely
to be explained by the balance of uptake and efflux of rosuvastatin
(Figure 1). In contrast, the rosuvastatin accumulation at pH 7.4 in
the presence of 5,7-DMF was small (Figure 1). Interestingly, the
increasing concentration of 5,7-DMF did not increase the initial
accumulation of rosuvastatin at 5 min and pH 7.4 (Figure 2). A
possible explanation for this phenomenon is that 5.7-DMF may be
a substrate of pH-sensitive transporter as in the case of rosuvastatin,
and the increase in 5,7-DMF concentration may strongly inhibit the
uptake of rosuvastatin at pH 7.4, because some substrates of ABC
efflux transporter are known to act as OATP2B1 substrates [9-11].
Involvement of the transporter in the uptake of 5,7-DMF in Caco-2
cells may be supported by the following that Papp A-B of 5,7-DMF in
Caco-2 cells (23.3 × 10-6 cm/s, [41]) is higher than that of rosuvastatin
(0.56 × 10-6 cm/s, [34]), in addition to the rapid absorption of 5,7-
DMF after oral administration [27]. Further study on the uptake
mechanism of 5,7-DMF in Caco-2 cells is necessary to explain the
little-increasing effect of 5,7-DMF on the rosuvastatin accumulation
at pH 7.4 (Figure 1 and 2). As in the case of P-gp [42], the increase
in pH is not likely to affect the rosuvastatin efflux mediated by BCRP
and MRP2 which use ATP as an energy source.
Varma et al. reported a higher accumulation of rosuvastatin in
Caco-2 cells at acidic pH [14]. They also reported that even in the
presence of rifamycin SV, an OATP2B1 inhibitor, the rosuvastatin
accumulation at acidic pH was higher in Caco-2 cells. This result
implies that a pH-sensitive transporter other than OATP2B1 could be
involved in rosuvastatin accumulation. Most likely is the involvement
of pH-sensitive transporter MCT1, as benzoic acid and pravastatin
decreased the rosuvastatin accumulation (Figure 5); benzoic acid and pravastatin are not only OATP2B1 substrates but also MCT1
substrates [7], rosuvastatin is the monocarboxylic acid like pravastatin
and atorvastatin, and the uptake of pravastatin and atorvastatin is
reported to be mediated by MCT1 [7]. The involvement of pH sensitive
accumulation of rosuvastatin by simple diffusion is likely to
be small as the pKa of rosuvastatin is 4.3 [20,43].
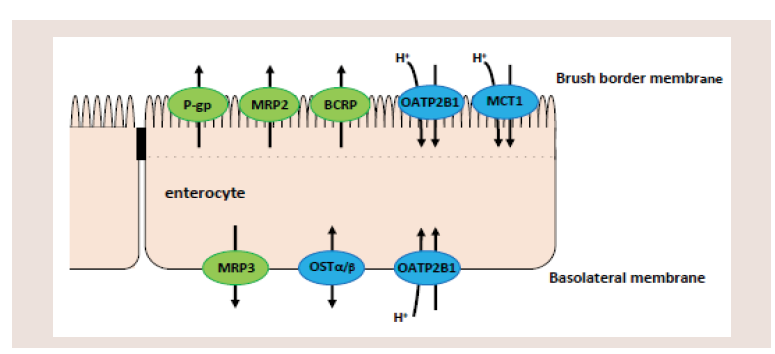
Figure 9 illustrates the membrane transporters related to
the transport of statins through brush border and basolateral
membranes: The inhibition of BCRP, MRP2 and/or P-gp by 5,7-DMF
could increase the A-to-B transport and the accumulation of statins,
whereas it could decrease the B-to-A transport with increases in the
accumulation [20,23]. Th e ratio (T/A) of basolateral exposure at 120
min in the absence of 5,7-DMF was very high (10.9) which suggests
the strong pumping of rosuvastatin mediated by BCRP and MRP2
using ATP energy (Table 1). The Papp A-B value of rosuvastatin in the
present study (0.24 × 10-6 cm/s) is similar to that reported previously
by Hua et al. [21] (0.261 × 10-6 cm/s), Li et al. [20] (0.25 × 10-6 cm/s)
and Fredlund et al. [34] (0.56 × 10-6 cm/s). On the other hand, the
Papp B-A values reported by Hua et al. [21] (0.594 × 10-6 cm/s), Li et al.
[20] (20.66 × 10-6 cm/s), the present study (5.13 × 10-6 cm/s) were all
higher than the Papa A-B values although these Papp B-A values vary widely.
Pravastatin (5 mM) resulted in a large decrease in the rosuvastatin
accumulation by 55% (Figure 5). Pravastatin appears to inhibit the
rosuvastatin uptake by OATP2B1 and MCT1, as pravastatin is the
substrate of both OATP2B1 and MCT1 [7]. Compared to rosuvastatin,
on the other hand, pravastatin may be sparingly secreted by the ABC efflux transporter(s), as the estimated Papp B-A value of pravastatin in
Caco-2 cells (difference between intrinsic-Papp A-B and Papp A-B values) is
markedly lower than that of rosuvastatin, whereas the Papp A-B values
of pravastatin and rosuvastatin are similar [34]. Thus, pravastatin
may strongly inhibit OATP2B1- and MCT1-mediated uptake of
rosuvastatin with a little inhibition of BCRP- and MRP2-mediated
effl ux of rosuvastatin, eventually, resulting in a marked decrease
of the rosuvastatin accumulation in Caco-2 cells by 55% (Figure 5). A great decrease in the rosuvastatin accumulation was found in
the incubation at 4°C (about 87%, Figure 6); the incubation at low
temperature appears to decrease the uptake of rosuvastatin not only
by carrier-mediated process of OATP2B1 and MCT1 but also by a
simple diffusion process.
Noteworthy is that the decreased rosuvastatin accumulation
by pravastatin and benzoic acid was greatly increased by 5,7-DMF,
which exceeded the control level of rosuvastatin accumulation in the
absence of these compounds (Figure 5). These phenomena suggest
that pravastatin and benzoic acid could act as the inhibitors of
OATP2B1 and MCT1, and 5,7-DMF may act as the inhibitor of BCRP
and MRP2.
The marked increase of rosuvastatin accumulation owing to ATP depletion
(2.4-fold) is similar to that owing to 5,7-DMF (2.0-fold)
(Figure 5), quercetin (2.0-fold) and BSP at 500 μM (1.8-fold) (Figure 5). Marked increases of the rosuvastatin accumulation could be
occurred by the depletion of cellular ATP content and the inhibition
of ATP-dependent efflux mediated by BCRP and MRP2.
Cyclosporin A is a non-specific inhibitor of OATPs and ABC
efflux transporters [44]. However, 100 μM cyclosporin A did not affect
the rosuvastatin accumulation in Caco-2 cells (Figure 5), probably by
the inhibitory balance between the uptake by OATP and the efflux
by BCRP and MRP2. Verma et al. [14] reported the concentration dependent
decreasing effect of cyclosporin A (up to 100 μM) on the
rosuvastatin accumulation in OTAP2B1-transfected HEK293 cells.
However, the expression of BCRP and MRP2 in these HEK293 cells
is unlikely. Contrary to in vitro studies, clinically used cyclosporin
A increases the rosuvastatin concentration in blood, probably as a
result of a decrease in hepatic accumulation of rosuvastatin by the
inhibition of OATP1B1 [12,13,43]. However, the exact inhibitory
effect of cyclosporin A on transporters is not clear, as cyclosporin
A is a potent inhibitor of several transporters including OATP1B1,
OATP2B1, OATP1B3, MRP2, BCRP, P-gp, etc. expressed in many
tissues.
Quercetin is known to inhibit many transporters such as OATPs
and MCT1 as well as the efflux transporters of P-gp, BCRP, and
MRP2 [45-47]. In this study, quercetin increased the rosuvastatin
accumulation 2.0-fold which is a similar extent of 5,7-DMF (2.0-
hold), 500 μM BSP (1.8-fold) and ATP depletion (2.4-fold) (Figure 5 and 6). In this study, quercetin is likely to increase the rosuvastatin
accumulation in Caco-2 cells as a result of the inhibition of P-gp and
BCRP rather than OATP2B1 and MCT1.
BSP and E3S are well-known substrates of OATP2B1 [1,3,7],
but they are reported to act as MRP2 inhibitor and BCRP inhibitor
[10,11], respectively. BSP at 100 and 500 μM concentrations in our
experimental conditions may act as the inhibitor of MRP2 rather than OATP2B1 [48], and eventually increased the rosuvastatin
accumulation (Figure 5). On the other hand, E3S at 100 μM
concentration may inhibit both OATP2B1-mediated uptake and
BCRP-mediated efflux, resulting in little change of the rosuvastatin
accumulation in Caco-2 cells. Ueno et al. (2012) reported that 500
μM BSP and 1000 μM E3S increased the accumulation of SN-38 (a
metabolite of irinotecan) in Caco-2 cells because of their inhibitory
effects on SN-38 efflux. To our knowledge, the rosuvastatin uptake
by OATP2B1 has not been clearly demonstrated in Caco-2 cells,
probably due to its strong efflux of rosuvastatin mediated by BCRP
and MRP2, and OATP2B1 substrates such as E3S and BSP act as
the inhibitors of not only OPTP2B1 but also MRP2 and BCRP. To
confirm the OATP2B1-mediated uptake (absorption) of statins,
studies have been undertaken using OATP2b1 transfected HEK293
cells and OATP2b1-deficient mice [14,17].
Studies are being undertaken on the transport of statins
through the basolateral membrane of enterocytes (Figure 9), and
proposed transporters related to statins are MRP3 [49], OSTα/β
[20], and OATP2B1 [23]. However, the transport of statins by these
transporters has not been confirmed by other researchers, especially
the existence of OATP2B1 at the basolateral membrane. In this study,
we used MK-571 and E3S as transporter inhibitors through the apical
membrane. However, MK-571 is known to activate the inhibitor of
MRP3 and E3S is known to be a substrate of OSTα/β [49,50]. Uptake
and efflux of statins in enterocytes, especially through the basolateral
membrane of rosuvastatin, are not fully understood.
Figure 9: Membrane transporters at brush border and basolateral membranes
of intestines involved statins absorption. P-gp: P-glycoprotein, MRP2 and
MRP3: multidrug resistance associated protein 2 and 3, BCRP: breast cancer
resistance protein, OATP2B1: organic anion transporting polypeptide 2B1,
MCT1: monocarboxylate transporter 1, OSTα/β: organic solute transporter α/β.
In conclusion, this study showed that 5,7-DMF increased
the uptake and the accumulation of rosuvastatin from the apical
membrane of Caco-2 cells which seems to be related to the inhibition
of rosuvastatin efflux mediated by BCRP and MRP2. The uptake of
rosuvastatin from the apical membrane may be mediated by not only
OATP2B1 but also MCT1. Some typical substrates of OATP2B1 did
not decrease the rosuvastatin accumulation, because they could act as
the substrates (inhibitors) of efflux transporters as well.