Journal of Pharmaceutics & Pharmacology
Download PDF
‘Diabète free: 160 mg garlic clove (Allium sativum), 180 mg cinnamonbark (Cinnamomumverum)
‘Pancréa free”: 100 mg ginseng root bark (Panax ginseng), 150 mg gurmar leaves (Gymnemasylvestre)
The test is used to measure the effect of the ‘Diabetes Free + Pancrea Free’ combination on basal blood glucose, i.e. in the absence of hypoglycaemia, with in order to predictits safety of use.
Research article
Hypoglycaemic Risk and Antihyperglycaemic activity of “Diabète free” and “Pancréas free”, an Anti-diabetic Combination from an Ivorian Traditional Practitioner
Etienne EK, Josué YK, Adebo AY, Awa S, GenevièveIrié NG and Giséle KS*
Department of Pharmacology, Faculty of biological and pharmaceutical sciences, University of Félix Houphouët-Boigny,
Abidjan - Côte d’Ivoire
Address for Correspondence:EFFO Kouakou Etienne, Department of Pharmacology, Faculty of biological and pharmaceutical sciences, University of Félix Houphouët-Boigny, Abidjan - Côte d’Ivoire, E-mail Id:
eff oet@yahoo.fr
Submission:15 September 2024
Accepted:12 October 2024
Published:15 October 2024
Copyright: © 2024Etienne EK, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Keywords:Diabetes; Diabète Free; Pancréa Free; Hypoglycaemic;
Antihyperglycaemic
Abstract
Diabetes remains a public health issue for which traditional
medicine offers a therapeutic response. The aim of this study was
to assess the hypoglycaemic risk and antihyperglycaemic activity
of ‘Diabète Free’ and ‘Pancréa Free’, remedies used by an Ivorian
traditional practitioner to treat diabetes.
For the hypoglycaemic risk, 4 groups of 6 rats per group were successively given ‘Diabète Free’ and ‘Pancréa Free’ by oral route at respective doses of 7mg/kg and 4.5mg/kg, 14mg/kg and 9mg/kg, 28mg/kg and 18mg/kg, or NaCl. To evaluate the antihyperglycaemic activity, 5 groups of 6 rats per group were successively treated with ‘Diabète Free’ and ‘Pancréa Free’ by oral route at respective doses of 7mg/kg and 4.5mg/kg, or 14mg/kg and 9mg/kg, or 28mg/kg and 18mg/kg, or NaCl, or glibenclamide at 10mg/kg. An oral glucose overload at a dose of 10g/kg was administered 30 min later. In each case, blood glucose levels were monitored every hour for 4 hours using blood from rats’ tailvein.
The ‘Diabète Free’ and ‘Pancréa Free’ combination showed no hypoglycaemic risk. The combination at doses of 14mg/kg and 9mg/ kg, as well as 28mg/kg and 18mg/kg, showed a signifi cant reduction in orally induced hyperglycaemia from the 1st hour without causing hypoglycaemia.
The combination of ‘Diabète Free’ and ‘Pancréa Free’ is thought to have antihyperglycaemic activity without anyrisk of hypoglycaemia. This remedy combination could be an alternative to conventional antidiabetic treatments.
For the hypoglycaemic risk, 4 groups of 6 rats per group were successively given ‘Diabète Free’ and ‘Pancréa Free’ by oral route at respective doses of 7mg/kg and 4.5mg/kg, 14mg/kg and 9mg/kg, 28mg/kg and 18mg/kg, or NaCl. To evaluate the antihyperglycaemic activity, 5 groups of 6 rats per group were successively treated with ‘Diabète Free’ and ‘Pancréa Free’ by oral route at respective doses of 7mg/kg and 4.5mg/kg, or 14mg/kg and 9mg/kg, or 28mg/kg and 18mg/kg, or NaCl, or glibenclamide at 10mg/kg. An oral glucose overload at a dose of 10g/kg was administered 30 min later. In each case, blood glucose levels were monitored every hour for 4 hours using blood from rats’ tailvein.
The ‘Diabète Free’ and ‘Pancréa Free’ combination showed no hypoglycaemic risk. The combination at doses of 14mg/kg and 9mg/ kg, as well as 28mg/kg and 18mg/kg, showed a signifi cant reduction in orally induced hyperglycaemia from the 1st hour without causing hypoglycaemia.
The combination of ‘Diabète Free’ and ‘Pancréa Free’ is thought to have antihyperglycaemic activity without anyrisk of hypoglycaemia. This remedy combination could be an alternative to conventional antidiabetic treatments.
Introduction
Diabetes is a major challenge for healthcare systems worldwide.
In 2021, approximately 537 million adults had diabetes, and this
figure is expected to rise up to 783 million by 2045 if appropriate
measures are not taken [1]. Type 1 diabetes and type 2 diabetes are
the most common forms of the disease [2].
In Côte d’Ivoire, the prevalence of diabetes in 2017 was
around 6.2% in the adult population [3]. This is due to a number
of factors, such as rapid urbanisation, changes in lifestyle, diet and
an aging population [4]. Diabetes-related complications, such as
cardiovascular disease, neuropathy and nephropathy, represent a
significant burden for Ivorian healthcare systems [5-7]. Therefore,
effective diabetes management requires an integrated approach
including lifestyle modifications, medication and, in some cases,
alternative therapies. Among the alternative approaches, herbal
remedies and natural products have increased in popularity. Around
80% of the population of Côte d’Ivoire rely on traditional medicine
for their healthcare, including the treatment of diabetes [8].
Studies have shown that several plants possess antihyperglycaemic
properties, making them potential candidates for diabetes management [9,10].
These traditional remedies are often chosen because of their accessibility, affordability and cultural
confidence[11]. However, the safety and efficacy of these plants are yet
to be scientifically demonstrated to ensure their safe use in modern
treatment protocols [12].
In this context, a combination of herbal remedies from an Ivorian
traditional practitioner called ‘Diabète Free’ and ‘Pancréa Free’
was the subject of an observational study on Ivorian diabetics, and
exhibited promising results [13]. However, no preclinical studies were
conducted on the efficacy and safety of this remedy combination.
The objective of this study was to evaluate the efficacy and safety of
thisanti-diabetic combination ‘Diabète Free’ and ‘Pancréa Free’.
Materials and methods
‘Diabète free’ and “Pancréa free” remedies:
The remedies were directly collected from the traditional
practitioner in April 2024. They are in the form of white tablets.
The partial composition given by the traditional practitioner was as
follows:‘Diabète free: 160 mg garlic clove (Allium sativum), 180 mg cinnamonbark (Cinnamomumverum)
‘Pancréa free”: 100 mg ginseng root bark (Panax ginseng), 150 mg gurmar leaves (Gymnemasylvestre)
Experimental animals:
Wistar rats (Rattus norvegicus) with an approximate 3 months
of age and weighing between 129 and 243 g.were used in this study.
They were kept in plexiglass cages with stainless steel lids and feeding
bottle. Temperature and humidity within the laboratory met the
standards of experimental premises (T= 22˚C ± 3˚C and 50-60%
humidity), with a 12 h of and 12 h of dark cycle. Animals were given
food pellets and had free access to water.Solvents and reagents:
Powders, solutions, solvents and standard substances used in this
study were as follows: Glucose powder (D glucose), Sodium chloride
solution (NaCl) 0.9%, Distilled water, Glibenclamide 5 mg (Daonil®)
(Laboratoire Sanofi (as standard substance).Equipment:
Several laboratory devices and equipment were used to carry out
this study, including scales, a glucometer, test strips, scalpel blades,
beakers, a mortar and pestle, a spatula, a watch glass, and syringes.Methods
Determination of equivalent dose in animals:
According to the owner of ‘Diabète Free’ and ‘Pancréa Free’
remedies, the recommended dose for treating diabetes in a 70 kg
adult is 1 tablet (160 mg of garlic) corresponding to 2.29 mg/kg/d for
‘Diabète Free’ and 1 tablet (100 mg of ginseng) corresponding to 1.43
mg/kg/d for ‘Pancréa Free’.Th e animal equivalent dose was estimated using the method of
Nair and Jacob [14].
For ‘Diabète free’:
The dose in experimental rats was obtained by multiplying the
human dose by a conversion factor, i.e. 2.29 x 6.2, giving a dose of
14mg/kg/d. Two other doses were used to bracket this equivalent
dose in animals, namely at 7mg/kg and 28mg/kg.A tablet containing 160mg of garlic extract from the « Diabète
Free »remedy was homogenised in 57ml of physiological water to
obtaina 2.8mg/ml solution and a volume of 10ml/kg was administered
to rats, corresponding to a dose of 28mg/kg. From this 2.8 mg/ml
stock solution, a dilution to one-half (½) was carried out to obtain
two solutions at 1.4 mg/ml and 0.7 mg/ml corresponding to doses of
14mg/kg and 7mg/kg respectively.
For ‘Pancréa free’:
Th e dose in experimental rats was obtained by multiplying the
human dose by the conversion factor, i.e. 1.43 x 6.2, giving a dose of
9mg/kg/d. Two other doses were used to bracket this equivalent dose
in animals, namely at 4.5mg/kg and 18mg/kg.A tablet containing 100mg of garlic extract from the ‘Pancréa
Free’remedy was homogenised in 55ml of physiological water to
obtain a 1.9mg/ml solution and a volume of10ml/kg, was given to
rats, corresponding to a dose of 19mg/kg. From this 1.9 mg/ml stock
solution, a dilution to one-half (½) was carried out to obtain two
solutions at 0.95 mg/ml and 0.475 mg/ml corresponding to doses of 9
mg/kg and 4.5 mg/kg respectively.
Glibenclamide 1mg/ml:
Four (4) tablets of glibenclamide 5 mg (Daonil®) were dissolved in
20 ml of physiological water for the preparation of the glibenclamide
1mg/ml solution. Corresponding to a dose of 10 mg/kg.Glucose solution 1000 mg/ml:
One hundred (100) grams of glucose in an hydrous powder form
were dissolved in 100 ml of physiological water to obtain the 1000 mg/
ml glucose solution corresponding to a dose of 10 g/kg.Assessment of hypoglycaemic risk:
Principle:The test is used to measure the effect of the ‘Diabetes Free + Pancrea Free’ combination on basal blood glucose, i.e. in the absence of hypoglycaemia, with in order to predictits safety of use.
Procedure:
Four (4) groups of homogeneous weight of 6 rats per group were
kept fasting 8 hours prior to experiment. Th e baseline blood glucose
levels of 24 rats were measured by collecting blood from the tailvein
and read with a glucometer ‘Hummas.
Animals were then given the diff erent solutions by oral route, at a
rate of 10 ml/kg, as follows: Group 1: rats received NaCl; Groups 2, 3
and 4: rats were successively given‘Diabète Free’ and ‘Pancréa Free’ at
doses of 7 mg/kg and 4.5mg/kg; 14 mg/kg and 9mg/kg; and 28 mg/kg
and 18mg/kg respectively.
After administration of substances, blood glucose levels were also
measured after 1h, 2h, 3h and 4h.
Assessment of antihyperglycaemic activity:
Principle:
The test is designed to measure the effect of ‘Diabètes Free +
Pancréa Free’ combination on oral hyperglycaemia caused by glucose
overload.
An antihyperglycaemic substance prevents hyperglycaemia in
rats. In the opposite case, hyperglycaemia is observed in rats.Procedure:
Five (5) groups of homogeneous weight of 6 rats per group were kept fasting 8 hours before experiment. Their baseline blood glucose levels were measured by collecting blood samples from the tailvein and then read with a glucometer‘ Hummas’.
Five (5) groups of homogeneous weight of 6 rats per group were kept fasting 8 hours before experiment. Their baseline blood glucose levels were measured by collecting blood samples from the tailvein and then read with a glucometer‘ Hummas’.
Animals were given the various solutions by oral route at a rate of
10ml/kg, as follows: Group 1: Rats received NaCl; Group 2: Rats was
administered glibenclamide at a dose of 10mg/kg; Groups 3, 4 and 5:
Rats successively received ‘Diabète Free’ and ‘Pancréa Free’ at doses
of 7 mg/kg and 4.5mg/kg; 14 mg/kg and 9mg/kg; and 28 mg/kg and
18mg/kg, respectively.
Aft er 30 minutes, they were all given a glucose overload of 10g/kg
and blood glucose levels were measured every hour for 4 hours, aft er
1, 2, 3 and 4 hours.
Data processing and analysis:
The results were expressed as the mean ± standard deviation.
Graphs were produced using Graph Pad Prism version 8.0.2 soft ware.
Means were compared by analysis of variance using the ANOVA test
at a risk of α = 5%.Results
Hypoglycaemic risk:
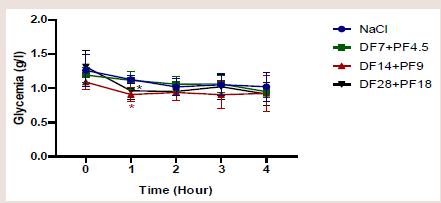
[Figure 1] shows the effect of the ‘Diabète Free et Pancréa Free’
combination at different doses on the baseline of blood glucose levels.
During the 4 hours of observation, the combination of ‘Diabète
Free’ at a dose of 7mg/kg and ‘Pancréa Free’ at a dose of 4.5mg/
kg did not cause hypoglycaemia compared to NaCl (p= 0.59). This
combination led to a marked hypoglycaemia after 1 hour at doses of
14mg/kg and 9mg/kg respectively (p=0.04); and 28mg/kg and 18mg/
kg (p=0.01) compared to NaCl. This hypoglycaemia was corrected
from the second (2nd)hour (P=0.30).Antihyperglycaemic activity:
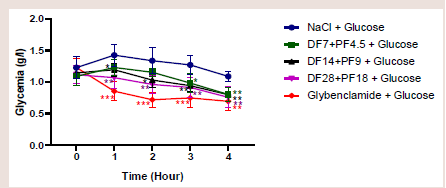
[Figure 2] shows the effect of combination of “Diabète Free
et Pancréa Free” at different doses on the prevention of oral
hyperglycemia.Glucose overload resulted in hyperglycaemia observed at the fi rst
(1st) hourin rats from the control group (NaCl). Th is hyperglycaemia
remained practically stable until the third (3rd) hour. However, it
began to decrease aft er the second (2nd) hour in rats given ‘Diabète
Free’ at a dose of 7mg/kg and ‘Pancréa Free’ at a dose of 4.5mg/kg.
Th is decrease was more observed at the third (3rd) hour (p=0.006) and
fourth (4th) hour (p=0.0003).
No increase in blood glucose levels was observed in rats given
‘Diabetes Free’ at a dose of 14mg/kg and ‘Pancrea Free’ at a dose of
9mg/kg despite oral glucose overload. Th e p values were 0.01, 0.01,
0.0007 and 0.00001 aft er 1h, 2h, 3h and 4h respectively.
A reduction in blood glucose despite glucose overload was
observed from the fi rst hour in rats given ‘Diabète Free’ at a dose of
Figure 2:Anti-hyperglycaemic effect of the combination of ‘Diabète Free and
Pancréa Free’, DF: Diabète Free; PF: Pancréa Free.
28mg/kg and ‘Pancréa Free’ at a dose of 18mg/kg. The p values were
0.004, 0.004, 0.002 and 0.001 after 1h, 2h, 3h and 4h respectively.
Basically, the ‘Diabète Free and Pancréa Free’ combination
tended to maintain blood glucose levels close to baseline, whereas
with glibenclamide, the antihyperglycaemic eff ect was followed by
hypoglycaemia compared to the control group (p<0.05).
Discussion
Th e aim of this study was to evaluate the hypoglycaemic risk and
antihyperglycaemic activity of the combination ‘Diabète free and
Pancréas free’, remedies from an Ivorian traditional practitioner.
‘Diabète free’ is said to contain garlic (Allium sativum) and
cinnamon (Cinnamomum verum), while “Pancréa free” contains
ginseng (Panax ginseng) and gurmar (Gymnema sylvestre).
Generally, the ‘Diabète free et Pancréa free’ combination did not cause hypoglycaemia during 4 hours of follow-up. However, the combination at doses of 14mg/kg and 9mg/kg and 25mg/kg and 18mg/ kg respectively showed a significant drop in baseline blood glucose levels during the first hour after administration. Th is hypoglycaemia was immediately corrected at the second (2nd)hour. According to the owner of these remedies, ‘Diabète Free’ has a hypoglycaemic effect and ‘Pancréa Free’ has a regenerative effect on the pancreas, enabling it to fulfi lits blood sugar regulation functions.
Generally, the ‘Diabète free et Pancréa free’ combination did not cause hypoglycaemia during 4 hours of follow-up. However, the combination at doses of 14mg/kg and 9mg/kg and 25mg/kg and 18mg/ kg respectively showed a significant drop in baseline blood glucose levels during the first hour after administration. Th is hypoglycaemia was immediately corrected at the second (2nd)hour. According to the owner of these remedies, ‘Diabète Free’ has a hypoglycaemic effect and ‘Pancréa Free’ has a regenerative effect on the pancreas, enabling it to fulfi lits blood sugar regulation functions.
In addition, the work of Wang et al.on the assessment and
selection of the hypoglycaemic risk of ginseng showed results similar
to our own. Wang et al. demonstrated that ginseng could significantly
reduce fasting glucose levels in diabetic rats without inducing a risk
of hypoglycaemia[15].
Similarly, the work of Sotaniemi et al.on ginseng [16] also showed similar results, confirming its role in regulating bloodsugar levels. Other studies, particularly on Gymnema sylvestre (gurmar), have shown that gurmar improves glucose balance and is involved in the repair and regeneration of pancreaticcells [17,18].
Th e eff ects of ‘Diabète free et Pancréa free’ combination could be explained by the presence of ginseng and gurmar. As for the antihyperglycaemic activity, the oral administration of a concentrated glucose solution (10 g/kg) to fasting animals produced hyperglycaemia from the 1st hour to the 3rd hour of follow-up. In response to this hyperglycaemia, the β cells of the islets of Langerhans are stimulated, leading to the secretion of insulin[19]. Th is hormone promotes glucose uptake by muscle cells, adipocytes and hepatocytes, causing the normalcy of blood glucose levels, as observed in control rats at the fourth hour. these results are similar to those obtained by Irié-N’Guessanet al.[20], who studied the antihyperglycaemic activity and hypoglycaemic risk of Sarenta, a medicinal plant remedy from the Ivorian pharmacopoeia. Irié-N’Guessanet al. [20] showed that oral administration of glucose at a dose of 5 g/kg in rats caused a peak in hyperglycaemia the first hour, followed by a decrease from the second (2nd)hour. This finding was also exhibited by Rasolofoson et al.[21]. However, difference in doses could justify the persistence of hyperglycaemia in this study.
Similarly, the work of Sotaniemi et al.on ginseng [16] also showed similar results, confirming its role in regulating bloodsugar levels. Other studies, particularly on Gymnema sylvestre (gurmar), have shown that gurmar improves glucose balance and is involved in the repair and regeneration of pancreaticcells [17,18].
Th e eff ects of ‘Diabète free et Pancréa free’ combination could be explained by the presence of ginseng and gurmar. As for the antihyperglycaemic activity, the oral administration of a concentrated glucose solution (10 g/kg) to fasting animals produced hyperglycaemia from the 1st hour to the 3rd hour of follow-up. In response to this hyperglycaemia, the β cells of the islets of Langerhans are stimulated, leading to the secretion of insulin[19]. Th is hormone promotes glucose uptake by muscle cells, adipocytes and hepatocytes, causing the normalcy of blood glucose levels, as observed in control rats at the fourth hour. these results are similar to those obtained by Irié-N’Guessanet al.[20], who studied the antihyperglycaemic activity and hypoglycaemic risk of Sarenta, a medicinal plant remedy from the Ivorian pharmacopoeia. Irié-N’Guessanet al. [20] showed that oral administration of glucose at a dose of 5 g/kg in rats caused a peak in hyperglycaemia the first hour, followed by a decrease from the second (2nd)hour. This finding was also exhibited by Rasolofoson et al.[21]. However, difference in doses could justify the persistence of hyperglycaemia in this study.
Pre-treatment with the combination of ‘Diabète Free et Pancréa
Free’ at doses of 14mg/kg and 9mg/kg, and 28mg/kg and 18mg/kg
respectively in rats, demonstrated a significant effect on reducing
the hyperglycaemic peak observed during glucose overload. The
combination of ‘Diabète Free et Pancréa Free’ therefore has an
eff ective preventive eff ect against orally-induced hyperglycaemia
in experimental rats. According to the traditional practitioner who
designed these remedies, ‘Diabète Free’ has antihyperglycaemic
properties and ‘Pancréa Free’ regenerates the pancreas, by stimulating
its blood sugar regulation functions. The combination of these
remedies might therefore has a synergistic effect against induced
hyperglycaemia.
These results are similar to those of Eidi et al.[22], who showed
that the oral administration of garlic extract significantly reduced
glycaemia while increasing serum insulin in rats, thus demonstrating
its antihyperglycaemic effects.
These results are also in line with studies conducted on
cinnamomum verum(cinnamon), where its antihyperglycaemic
activity has been demonstrated [23].
Since garlic and cinnam on are components of the ‘Diabète Free’
and ‘Pancréa Free’ remedies, the antihyperglycaemic activity of the
combination could be justified by the presence of these plants.
The hypothesis of the mechanism of action of these remedies
could be explained by the ability to improve ATP production and
consequently increase insulin production following the upgrading of
pancreatic β-function and also the reduction in insulin resistance as
demonstrated with ginseng [24]. This mechanism might justify the
absence of hypoglycaemic risk in the ‘Diabètes Free and Pancréas Free’
combination, which can be observed in contrast with glibenclamide,
which is a purelyinsulin-secreting agent.
The combination of ‘Diabète Free et Pancréa Free’ could therefore
be of good interest in both type 1 and type 2 diabetes, with good safety
of use.
Conclusion
The objective of this study was to evaluate the efficacy and safety
of ‘Diabète Free’ and ‘Pancréas Free’, two remedies combined by
an Ivorian traditional practitioner for the treatment of diabetes, in
experimental animals.
The animal equivalent dose was 14 mg/kg for ‘Diabète Free’ and
9 mg/kg for ‘Pancréa Free’. Two other doses were used to bracket
this animal equivalent dose, namely at 7mg/kg and 28mg/kg for
‘Diabète Free’ and 4.5mg/kg and 18mg/kg for ‘Pancréa Free’. We
found that the combination of ‘Diabète Free’ and ‘Pancréa Free’ did
not show any hypoglycaemic risk apart from hyperglycaemia. As for
the antihyperglycaemic activity, this combination demonstrated
antihyperglycaemic activity in experimental rats.
This finding highlights the therapeutic potential of this remedy in the management of diabetes mellitus and suggests that it could represent an effective medication to conventional treatments.
Conflict of Interest: We declare no conflicts of interest. Funding sources: This research didn’t receive grants from any funding agency in the public, commercial or not-for-profi t sectors.
This finding highlights the therapeutic potential of this remedy in the management of diabetes mellitus and suggests that it could represent an effective medication to conventional treatments.
Conflict of Interest: We declare no conflicts of interest. Funding sources: This research didn’t receive grants from any funding agency in the public, commercial or not-for-profi t sectors.