Journal of Surgery
Download PDF
Case Report
*Address for Correspondence: Shahid Habib MD, University of Arizona Health Sciences Center, 1501 N Campbell Avenue, Tucson, Arizona, USA 85724, E-mail: shabib@surgery.arizona.edu
Citation: Habib S, Chang CCH, De Vera M, Rana AA, Shaikh OS. Interferon Treatment Improves Survival among Liver Transplant Recipients with Recurrent Hepatitis C. J Surgery. 2013;1(2): 6.
Copyright © 2013 Habib S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 2
Submission: 02 July 2013 | Accepted: 04 September 2013 | Published: 09 September 2013
Reviewed & Approved by: Dr. Waldo Concepcion, Department of Surgery, Stanford University School of Medicine, USA
The vast majority of patients were placed on tacrolimus based immunosuppressive regimens and few received induction immunosuppression with either anti-thymocyte globulin (Thymoglobulin®) or alemtuzumab (Campath®). The proportion of patients who received tacrolimus was higher in the treatment group, and patients also received higher average daily tacrolimus dosage compared to the no-treatment group. The use of either intravenous or oral corticosteroid was similar in the two groups, but recipients in the treatment group more often received a cumulative steroid dosage of >1 gram. Steroid bolus use for acute rejection episodes was similar in both groups. The treatment group was more likely to have higher serum ALT and AST levels and lower serum alkaline phosphatase levels at 6 months post-transplant, and less likely to have a cholestasis index of ≤ 2. Two hundred and sixty-four patients received either interferon monotherapy (n=47, 18%) or combination therapy (n=217, 82%). The mean and median duration of treatment were 73 and 58 weeks, respectively. Mean and median times to initiation of treatment were 61 and 40 weeks from time of transplantation. Majority of patients (> 75%) initiated treatment within 6-18 months of transplantation. The treatment group was more likely to have higher serum ALT and AST levels and lower serum alkaline phosphatase levels at 6 months post-transplant, and less likely to have a cholestasis index of ≤ 2. Biochemical markers at one year post transplantation showed higher AST and ALT in treatment group whereas alkaline phosphatase was higher in no-treatment group. Bilirubin and creatinine were not different between treatment and no-treatment group at 12 months post transplantation. Liver biopsies (85% of treatment group) demonstrated that the Ishak-Knodell fibrosis score at initiation (+/-3 months) of treatment was 1.49. Only 1/3rd (30%) of the no treatment group had liver biopsies done at or around the same time interval (12 months +/-3 months) which demonstrated a fibrosis score of 1.40.
Multivariable analysis
Interferon Treatment Improves Survival among Liver Transplant Recipients with Recurrent Hepatitis C
Shahid Habib1*, Chung-Chou H. Chang2, Michael De Vera3, Abbas A Rana4 and Obaid S. Shaikh5
- 1Divisions of Gastroenterology & Hepatology and Transplantation, University of Arizona, Arizona, USA
- 2Department of Biostatistics, Graduate School of Public Health, University of Pittsburgh, Pennsylvania, USA
- 3Division of Transplantation Surgery, Loma Linda University Medical Center, California, USA
- 4Division Transplantation Surgery, University of Arizona, Arizona, USA
- 5Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh, Pennsylvania, USA
*Address for Correspondence: Shahid Habib MD, University of Arizona Health Sciences Center, 1501 N Campbell Avenue, Tucson, Arizona, USA 85724, E-mail: shabib@surgery.arizona.edu
Citation: Habib S, Chang CCH, De Vera M, Rana AA, Shaikh OS. Interferon Treatment Improves Survival among Liver Transplant Recipients with Recurrent Hepatitis C. J Surgery. 2013;1(2): 6.
Copyright © 2013 Habib S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 1, Issue: 2
Submission: 02 July 2013 | Accepted: 04 September 2013 | Published: 09 September 2013
Reviewed & Approved by: Dr. Waldo Concepcion, Department of Surgery, Stanford University School of Medicine, USA
Abstract
Hepatitis C in the liver allograft recipient has an aggressive course. A significant proportion of such recipients develop graft fibrosis and cirrhosis within five years of transplantation. Treatment efficacy with standard or pegylated interferon and ribavirin is suboptimal compared to the immunocompetent individuals. However, the effect of such therapy on graft and patient survival remains unknown.Objectives: To determine the efficacy of interferon based therapies in liver transplantation recipients with recurrent hepatitis C, and to determine the effect of interferon treatment on graft and patient survival.
Methods: We retrospectively analyzed 823 hepatitis C patients, who underwent liver transplantation at Thomas E Starzl Transplantation Institute, University of Pittsburgh between January 1992 and April 2006. Two hundred and sixty four patients received either interferon (all kinds) monotherapy, or combination therapy. The primary end point was graft failure and death.
Results: Both treated and untreated groups were similar in clinical characteristics at the time of transplantation. In treatment group, 77% received prednisone based immunosuppression as compared to 75% in no treatment group. 57% of patients in treatment group had HCV RNA >1 million units/ml after transplantation. Patients were categorized into four groups; no treatment (n=440), ≤ 24 weeks of treatment (n=38), 25-48 weeks of treatment (n=64) and >48 weeks of treatment (162). Total bilirubin > 2 at 6 and 12 months and an AST/ALT ratio of >1 at 6 month of transplantation are independently associated with poor patient survival. Patients who received treatment for >48 weeks have significantly improved survival independent of sustained viral response (HR 0.33, p=< 0.001).
Conclusion: Among liver transplant recipients with recurrent hepatitis C, treatment with any interferon with or without ribavirin for >48 weeks is significantly associated with improved patient survival regardless of viral response. Prospective trials are indicated to confirm these findings.
Keywords
SHCV; Outcome; Prognosis; Graft survival; Patient survival; Liver allograft; Fibrosis progression; Interferon; RibavirinAbbreviations
MELD: Model for Endstage Liver Disease; DHHS: Department of Health and Human Services; TIPS: Transjugular Intrahepatic Portosystemic Shunt; UNOS: United Network of Organ Sharing; EDIT: Electronic Data Interface for Transplantation; HIV: Human Immunodeficiency VirusIntroduction
Most liver transplant recipients with chronic hepatitis C have an accelerated disease course resulting in inferior cumulative graft and patient survival compared to the non-HCV infected recipients [1,2]. In some HCV patients, severe cholestatic hepatitis develops that results in rapid graft failure [3,4]. A number of viral, host and donor factors have been implicated in disease progression [5]. Such factors include a more advanced donor age, high pre-transplantation serum HCV RNA level, frequency of rejection episodes and the use of bolus corticosteroids. Until now, treatment of recurrent hepatitis C is disappointing in regards to sustained viral response (SVR) rates as most patients either fail to tolerate or respond to the standard combination treatment with pegylated interferons and ribavirin [5,6]. Most of the published data consist of small studies. There has been difficulty enrolling patients in prospective randomized trials to determine efficacy of such treatment in HCV liver transplant recipients [7]. Only one study has investigated graft survival after treatment with pegylated interferon and ribavirin treatment [8]. Moreover, the patient survival benefit of such treatment is unknown.We followed a large cohort of patients transplanted for HCV related liver disease. Interferon based therapy is routinely considered for all patients with recurrent hepatitis once the initial post-operative recovery phase is complete. We determined the effect of interferon based treatment on graft and patient survival compared to those who did not receive treatment among recipients with recurrent hepatitis C.
Patients and Methods
Study populationAll adult patients (18 years and older) transplanted for HCV disease between January 1992 and April 2006 were included in the study. Patients were identified by an electronic search of the liver transplantation database that prospectively enrolled transplant candidates and recipients. The study included primary transplantations alone, but grafts with primary non-function were disregarded. Therefore, the first functioning organ was considered the primary allograft. Patients without available allograft biopsies were excluded from the analysis. Recipient, donor, viral and transplant related variables were analyzed (Table 1). Patients were categorized into the treatment and non-treatment groups, and the treatment group was further categorized into three subgroups based on the duration of interferon treatment: 1) < 24 weeks of treatment, 2) 25-48 weeks of treatment and 3) > 48 weeks of treatment. Treatment was considered in all patients with recurrent HCV infection based on abnormal LFTs and histological evidence of recurrence. Interferon therapy included unmodified interferon-α2b (Intron-A®), pegylated interferon-α2b (Peg-Intron®) and pegylated interferon-α2a (Pegasys®) with or without ribavirin. Length of treatment varied because of side effects and response to treatment. No treatment group comprised of patients, who refused to take treatment because of higher risks and low probability of SVR with treatment. Patients who received less than 12 weeks of therapy were included in the non-treatment group. These are patients who did not tolerate treatment because of side effects of treatment. Genotype 2 and 3 patients received treatment for six months. In patients with other genotypes, treatment was planned for at least 48 weeks. In these patients, treatment was discontinued prematurely because of significant side effects and/or non-response to treatment. Patients that received treatment for greater than 48 weeks included repeated courses of treatment with periods of no treatment and continued treatment without interruptions.
Definitions
Patient survivalWe defined patient survival as the interval between liver transplantation and death or end of follow.
Graft Survival
It was defined as interval between transplantation and graft loss or end of follow up.
Graft loss
Graft loss was defined by either re-transplantation or patient’s death.
HCV diagnosis
The diagnosis of hepatitis C was based on a positive anti-HCV test and/or HCV RNA. All other patients were categorized to have non-HCV disease.
Sustained viral response (SVR)
Patients were considered to have sustained viral response if HCV RNA was negative at 6 months after completion of treatment.
Cholestasis index
We calculated cholestasis index by determining the ratio between serum ALT level/upper limit of normal for serum ALT and serum alkaline phosphatase level/upper limit of normal; an index of ≤ 2 was considered consistent with cholestasis.
Indication of treatment
There were no well defined indications for treatment; however, patients were considered for treatment based on clinical need as defined by abnormal liver injury tests and/or histological recurrence with evidence of progressive disease. As a general policy at our center, treatment is offered to all genotype 2 and 3 patients post transplant, whereas in genotype 1 patients, treatment is offered only with progressive disease on liver histology such as MHAI grade of 3-4 and fibrosis stage of ≥ 1 (Modified Ishak-Knodell activity Index).
Study endpoints
Our endpoints were the effect of interferon therapy on graft and patient survival.
Statistical analysis
Chi-square and Mann-Whitney U tests were used to compare baseline clinical features for dichotomous and continuous variables respectively. Kaplan-Meier and Cox proportional hazards regression model were used for univariate and multivariable analyses, respectively. Only variables statistically significant on univariate analysis were included in the multivariable regression model. A p-value of ≤ 0.05 was considered statistically significant. The Kaplan-Meier survival curves were tested for statistical significant difference with the Peto-test, not the standard Log-Rank test. The Peto-test places more emphasis on the data at the beginning of the survival curve where the proportion of cases at risk is larger using a weighted mean of the observed minus the expected result (Kleinbaum, D.G., Survival Analysis, NY: Springer-Verlag Inc., 1996).
Results
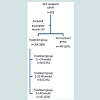
Clinical featuresDistribution of study cohort is shown in Figure 1. During the study period of 14 years, a total of 823 patients underwent liver transplantation for cirrhosis due to HCV. One hundred and eight patients were excluded because of incomplete information - mainly a lack of allograft biopsies. In 11 patients, treatment duration was not clearly defined. After exclusions, the study population was comprised of 704 recipients that included 264 (38%) patients treated with interferon for recurrent HCV and 440 (62%) patients who were not treated. The treatment group of 264 recipients included 38 (14%) treated for ≤ 24 weeks (Group 1), 64 (24%) treated for 25-48 weeks (Group 2) and 162 (62%) who received >48 weeks of treatment (Group 3). Most demographic and clinical features were similar in the no-treatment and treatment groups (Table 1). The majority of patients were white males with a mean age of 51 years at the time of transplantation. Six percent of the recipients (43/704) were older than 65 years, with a higher proportion in the no-treatment group. Donor features were also similar in the two groups, but donors were relatively younger with a mean age of 41 years in the treatment group. However, one-third of the donors were older than 50 years and almost one-half were female. The severity of liver disease as designated by the Child-Pugh scores and MELD scores was also similar. We also looked at the year of transplantation and categorized patients according to the applicable organ allocation criteria. Recipients were equally distributed between the pre-Child-Pugh, Child-Pugh and MELD era, and the proportion of patients who received treatment were similar in the three era (p=0.2). Most recipients in both groups had HCV genotype 1 but the treated group had a higher proportion of patients with serum HCV RNA level of >1 million units/ml. Operative factors such as the mean warm ischemia time was similar in both groups but cold ischemia time was longer in the no-treatment group.
The vast majority of patients were placed on tacrolimus based immunosuppressive regimens and few received induction immunosuppression with either anti-thymocyte globulin (Thymoglobulin®) or alemtuzumab (Campath®). The proportion of patients who received tacrolimus was higher in the treatment group, and patients also received higher average daily tacrolimus dosage compared to the no-treatment group. The use of either intravenous or oral corticosteroid was similar in the two groups, but recipients in the treatment group more often received a cumulative steroid dosage of >1 gram. Steroid bolus use for acute rejection episodes was similar in both groups. The treatment group was more likely to have higher serum ALT and AST levels and lower serum alkaline phosphatase levels at 6 months post-transplant, and less likely to have a cholestasis index of ≤ 2. Two hundred and sixty-four patients received either interferon monotherapy (n=47, 18%) or combination therapy (n=217, 82%). The mean and median duration of treatment were 73 and 58 weeks, respectively. Mean and median times to initiation of treatment were 61 and 40 weeks from time of transplantation. Majority of patients (> 75%) initiated treatment within 6-18 months of transplantation. The treatment group was more likely to have higher serum ALT and AST levels and lower serum alkaline phosphatase levels at 6 months post-transplant, and less likely to have a cholestasis index of ≤ 2. Biochemical markers at one year post transplantation showed higher AST and ALT in treatment group whereas alkaline phosphatase was higher in no-treatment group. Bilirubin and creatinine were not different between treatment and no-treatment group at 12 months post transplantation. Liver biopsies (85% of treatment group) demonstrated that the Ishak-Knodell fibrosis score at initiation (+/-3 months) of treatment was 1.49. Only 1/3rd (30%) of the no treatment group had liver biopsies done at or around the same time interval (12 months +/-3 months) which demonstrated a fibrosis score of 1.40.
A total of 190 (26%) recipients died in study period, with 22% the deaths attributed to liver or graft failure. In the no treatment group, 47% patients died, with only 13% of the deaths attributed to liver or graft failure. In treatment group, 21% of patients died with 25% dying from graft failure.
Survival analysis
Overall, the cumulative graft survival at 1, 5, and 10 years of transplantation was 80%, 65% and 53% and patient survival was 83%, 68% and 54%, respectively. Graft survival at 1, 5 and 10 years post-transplant in the treatment group (94%, 89% and 64%), was significantly higher (p < 0.0001) compared to no treatment group (71%, 53%, and 41%). Patient survival (Figure 2) at 1, 5, and 10 years of transplantation was 97%, 86% and 69%, respectively; whereas in the no-treatment group it was 74%, 56% and 45%, respectively (p < 0.0001). Both patient and graft survival were better in patients who received longer duration of treatment (Figure 3). In treatment group, 117 patients (42%) achieved PCR negative status during the course of treatment. Among PCR negative patients, SVR status was confirmed in 57 patients. In other patients, we were unable to establish SVR status because of incomplete data since actual SVR rate could not be calculated. Patients with SVR had improved survival as compared to patients with PCR negative status (Figure 4). Patients that achieved PCR negative or SVR status had improved patient survival as compared to untreated patients (Figure 4). Because of retrospective nature of this analysis, further details regarding side effect profiles and supportive treatments were unavailable.
Multivariable analysis
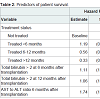
Only longer duration (> 48 weeks) of treatment (mono/combination) was independently associated with improved graft (HR 0.3, p=< 0.001) and patient survival (HR 0.33, p=< 0.001) (Figure 2). The impact of a number of variables on patient and graft survival was examined (Table 1). Independent predictors of poor graft and patient survival included serum total bilirubin levels of >2 at 6 and 12 months post-transplant and AST/ALT ratio >1 at 6 months (Table 2).
Discussion
Treatment of hepatitis C in the transplant recipient has been based on the clinical experience in the non-immunosuppressed, non-transplanted population. A well designed, controlled study is difficult to apply to the transplant population because of the concurrent hematologic and renal effects of immunosupppression. Most transplant centers address the risk of recurrent HCV by reducing immunosuppressive therapy and treating rejection episodes cautiously. Recurrent HCV is treated directly if it is associated with significant histologic liver injury. Among such patients, several uncontrolled studies have examined the efficacy of unmodified interferon and ribavirin monotherapies, as well as, that of interferon and ribavirin combination [5]. Experience with pegylated interferon in combination with ribavirin in the post-transplantation setting is limited [13-25].The current study demonstrated important findings in relation to HCV treatment and its effect on survival in the posttransplant setting. To our knowledge, this is the first report of this kind. Overall patient survival in this large cohort of HCV positive liver allograft recipients was similar to previous reports [2]. It is evident from these results that treating recurrent hepatitis C in liver transplant recipients is beneficial. It prolongs patient survival with or without achieving HCV RNA negativity or sustained virologic response. Patients who were treated for more than six months had better survival; however, longer duration of treatment (>12 months) had better outcomes compared to 6-12 months of treatment.
Elevated serum total bilirubin, AST and ALT levels or AST/ALT ratio greater than 1 at six months of transplantation were poor prognostic factors for patient survival. These findings suggest aggressive recurrent HCV infection in the absence of other etiological factors. All such patients warrant liver biopsies to establish histological recurrence. Treatment should then be considered with interferon based treatment with or without ribavirin. The lower rate of treatment in this group of patients suggests that the likely presence of contraindications to treatment. Another interesting finding is the higher likelihood of treatment in the 2002-2006 era as compared to previous eras. Still, the rate of progression to cirrhosis was higher during this time period, a finding that is consistent with other reports [1]. The underlying reasons remain unclear. One of the short comings of this retrospective analysis is the timing of treatment in relation to transplantation and last liver biopsy remains unknown. Since 2011, triple therapy is standard of care for HCV infection in the pretransplant setting, but its utility in post transplant setting is yet to be established.
In conclusion, all patients with recurrent HCV infection need to be considered for treatment with pegylated interferon and ribavirin for at least 48 weeks. In patients, who fail to achieve SVR, longer duration of treatment should be considered. The optimal timings and indications for treatment and the duration of treatment remain to be established. It may be that treatment needs to tailor to individualized viral kinetics.
Acknowledgements
Authors wish to thank Ed Mister and Sana Shahid for their help in data collection, analysis and preparation of manuscript.References
- Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, et al. (2000) HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol 32: 673-684.
- Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR (2002) The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 122: 889-896.
- Schluger LK, Sheiner PA, Thung SN, Lau JY, Min A, et al. (1996) Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 23: 971-976.
- Doughty AL, Spencer JD, Cossart YE, McCaughan GW (1998) Cholestatic hepatitis after liver transplantation is associated with persistently high serum hepatitis C virus RNA levels. Liver Transpl Surg 4: 15-21.
- Shaikh OS (2007) Hepatitis C Virus Infection Following Liver Transplantation: Natural History and Treatment. In: Medscape Today.
- Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K (2006) Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant 6: 1586-1599.
- Shakil AO, Habib S, Berk B, Eghtesad B, Marcos A, et al. (2004) A trial of pegylated interferon and ribavirin among liver transplant recipients with hepatitis C: reasons for screening failure and low enrollment. University of Pittsburgh, Pittsburgh, PA. Hepatology 40: 360.
- Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, et al. (2008) Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant 8: 2426-2433.
- Akamatsu N, Sugawara Y (2012) Liver transplantation and hepatitis C. Int J Hepatol 2012: 686135.
- Rodriguez-Luna H, Khatib A, Sharma P, De Petris G, Williams JW, et al. (2004) Treatment of recurrent hepatitis C infection after liver transplantation with combination of pegylated interferon alpha2b and ribavirin: an open-label series. Transplantation 77: 190-194.
- Neff GW, Montalbano M, O’Brien CB, Nishida S, Safdar K, et al. (2004) Treatment of established recurrent hepatitis C in liver-transplant recipients with pegylated interferon-alfa-2b and ribavirin therapy. Transplantation 78: 1303-1307.
- Dumortier J, Scoazec JY, Chevallier P, Boillot O (2004) Treatment of recurrent hepatitis C after liver transplantation: a pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol 40: 669-674.
- Heydtmann M, Freshwater D, Dudley T, Lai V, Palmer S, et al. (2006) Pegylated interferon alpha-2b for patients with HCV recurrence and graft fibrosis following liver transplantation. Am J Transplant 6: 825-833.
- Castells L, Vargas V, Allende H, Bilbao I, Luis Lazaro J, et al. (2005) Combined treatment with pegylated interferon (alpha-2b) and ribavirin in the acute phase of hepatitis C virus recurrence after liver transplantation. J Hepatol 43: 53-59.
- Neumann U, Puhl G, Bahra M, Berg T, Langrehr JM, et al. (2006) Treatment of patients with recurrent hepatitis C after liver transplantation with peginterferon alfa-2B plus ribavirin. Transplantation 82: 43-47.
- Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, et al. (2005) Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology 41: 289-298.
- Oton E, Barcena R, Moreno-Planas JM, Cuervas-Mons V, Moreno-Zamora A, et al. (2006) Hepatitis C recurrence after liver transplantation: Viral and histologic response to full-dose PEG-interferon and ribavirin. Am J Transplant 6: 2348-2355.
- Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, et al. (2011) PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. LiverTranspl 17: 528-538.
- Cimsit B, Assis D, Caldwell C, Arvelakis A, Taddei T, et al. (2011) Successful treatment of fibrosingcholestatic hepatitis after liver transplantation. Transplant Proc 43: 905-908.
- Tamura S, Sugawara Y, Yamashiki N, Kaneko J, Kokudo N, et al. (2010) Pre-emptive antiviral therapy in living donor liver transplantation for hepatitis C: observation based on a single-center experience. Transpl Int 23: 580-588.
- Schmidt SC, Bahra M, Bayraktar S, Berg T, Schmeding M, et al. (2010) Antiviral treatment of patients with recurrent hepatitis C after liver transplantation with pegylated interferon. Dig Dis Sci 55: 2063-2069.
- Jain A, Sharma R, Ryan C, Safadjou S, Kashyap R, et al. (2010) Response to antiviral therapy in liver transplant recipients with recurrent hepatitis C viral infection: a single center experience. Clin Transplant 24: 104-111.
- Dinges S, Morard I, Heim M, Dufour JF, Mullhaupt B, et al. (2009) Pegylated interferon-alpha2a/ribavirin treatment of recurrent hepatitis C after liver transplantation. Transpl Infect Dis 11: 33-39.
- Berenguer M, Roche B, Aguilera V, Duclos-Vallee JC, Navarro L, et al. (2013) Efficacy of retreatment of hepatitis C virus infection after liver transplantation: role of aggressive approach. LiverTranspl 19: 69-77.
- Akamatsu N, Sugawara Y (2012) Liver transplantation and hepatitis C. Int J Hepatol 2012: 686135.