Journal of Surgery
Download PDF
Research Article
Old Dogs and New Techniques: Comparing Complete Robotic Adoption to Laparoscopic Surgery-A Single Institution Experience with Distal Pancreatectomies
Carter M. Powell BS1, , Christine MG Schammel2 and Steven D. Trocha3*
1Kenyon College, Gambier OH 43022, USA
2Pathology Associates, Department of Pathology, Greenville SC 29605,
USA
3Department of Surgery, Greenville Health System, Greenville SC
29605, USA
*Address for Correspondence:
Steven D.Trocha, MD,FACS Chief, GI Liver Division, Department of Surgery ,
Prisma Health, Upstate 900 W Faris, Greenville SC 29605, USA Phone: 864-
455-1200 Fax:864-455-1209 E-mail : Steve.trocha@prismahealth.org
Submission: 26 January, 2023
Accepted: 27 February 2023
Published: 02 March, 2023
Copyright: © 2023 Carter M. Powell BS, et al. This is an open access
article distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
The laparoscopic distal pancreatectomy (LDP) is superior to
the open approach; however, proximal dissection, hand-assisted
(HA) approaches and conversion to open resection can be
improved upon. Robotic distal pancreatectomy (RDP) addresses the
limitations of LDP with better optics (3D), magnification, instrument/
visual stabilization and dexterity of the instrumentation. We sought
to investigate RDP vs. LDP and to introduce a new variable, tumor
distance from the superior mesenteric vein (SMV), to assess how
proximal the dissection was performed. A consecutive sample
of 45 patients who underwent minimally invasive distal pancreas
resection between 2/1/2012 to 6/30/2018 was completed. Typical
demographics and clinicopathologic variables were collected,
including outcomes. Overall, 22 LDPs and 23 RDPs, were evaluated.
No demographics, comorbidities, or ASA score were significantly
different between the cohorts. Neither differences in tumor size (LDP:
3.4cm +/- 2.8, RDP: 3.1cm +/- 1.9; p=0.80) or distance from the SMV
(LDP: 4.1cm +/- 3.0, RDP: 3.9 cm+/- 2.9; p = 0.89) were significantly
different. Positive margins were similar between groups; lymph nodes
were less with LDP than RDP (mean 6.4 and 10, respectively; p=0.09).
Post-operative complications and length of stay (mean 5.4 and 5.3
days, respectively) were similar between groups (p=0.27; p=0.94).
We show that converting to an entirely robotic approach for distal
pancreatectomies is safe, effective, with potentially better lymph
node dissection and a learning curve that demonstrates adoption at
any level of post residency training. Additionally, tumor distance from
the SMV/portal vein confluence could help quantify the theoretical
technical advantages of robotic distal pancreatectomy.
Keywords
Robotic distal Pancreatectomy; Adoption of New
Technology; Pancreatectomy; Robotic resection
Introduction
A complex and challenging procedure, distal pancreatectomy
(DP) has traditionally been performed via an open approach [1].
With advancements in technology, the first laparoscopic distal
pancreatectomy (LDP) was performed in 1994 by Cuschieri [2].
Compared to open surgery, LDP is associated with a reduction in
hospital stay, analgesic requirements, and blood loss [3-5]. Despite
the benefits of a minimally invasive approach, LDP has limitations.
The presence of large vascular structures, the retroperitoneal location,
and the concern for an inadequate margin clearance create obstacles
for surgeons who choose LDP, sometimes forcing them to convert to
a HA or open approach [5,6].
Robotic surgery represents the latest innovation in minimally
invasive surgery. Melvin et al. reported the first robotic distal pancreatectomy (RDP) in 2003, the same year Giulianotti et al.
published their series of robotic pancreas resections [7,8]. Robotic
surgery has allowed surgeons to overcome the limitations of LDP,
while maintaining a minimally invasive approach. Most notably, a
robotic approach provides a larger range of motion due to an internal
articulated endo-wrist. Robotics also offer a three-dimensional highdefinition
surgical view and tremor filtration, which can significantly
improve ergonomics for the surgeon [9]. Theoretically, these
technical advantages should afford the surgeon greater precision,
possibly providing them more access to tumors that would be not
considered for a laparoscopic approach and thus relegated to a HA
or open method.
Despite the benefits of robotic surgery, the adoption of RDP has
been slow. For veteran surgeons, adopting new surgical approaches
can be daunting. This hesitation is often due to the loss of tactile
feedback with robotics, which relies on “visual haptics,” and concerns
over increased operating time during the early learning curve as
well as possible increased cost associated with robotic surgery [1].
Furthermore, the adoption of a new surgical approach can be hindered
by a lack of training/experience, comfort with old approaches, and
concerns regarding outcomes. A growing body of literature has arisen
to compare the outcomes of LDP and RDP [1,5,10-13]. Meta-analyses
by Gavriilidis (2016) and Zhou (2016) investigate the findings of 8
retrospective studies and 2 prospective studies comparing LDP and
RDP [14,15]. RDP was found to be a safe and comparable alternative
to LDP, with no differences found between the two approaches.
The aim of this study was to investigate the effectiveness of
converting to an entirely robotic platform for distal pancreatectomies
by a veteran surgeon (old dog) and to introduce a new variable, tumor
distance from the superior mesenteric vein (SMV), as a measure of
precisely assessing how proximal the dissection was performed.
Methods
Following IRB approval, a retrospective analysis from 2/1/2012
to 6/30/2018 of 45 consecutive patients who underwent minimally
invasive distal pancreas resection at our tertiary institution was completed. Patients with multiple other operations, those without
followed up at our institution, and patients for whom complete
records were not available were excluded. All procedures were
performed by a single surgeon. The LDP population consisted
of patients who received treatment before robotic resection was
available at our institution (2/1/2012 to 4/30/2016). The LDP cohort
included those resections that involved hand assistance as well as
those requiring conversion to HAL. The RDP population consisted
of patients treated following the introduction of robotic resection
after 4/30/2016. This cohort included patients resected with robotic
technology only and those surgeries that were converted from
robotics to open resection.
Robotic surgeries were completed using the DaVinci® Robotics
(Intuitive, Sunnyvale, CA).
Preoperative imaging noted tumor size and distance from
the SMV/portal vein confluence as a comparison of the perceived
the difficulty of the resection and appropriate use of the surgical technique
(LDP+/- HAL; RDP conversion to open). For the purposes of our
study, we considered any patient that was started or changed to HAL
as an indication of a case outside a straight MIS approach.
All data were retrospectively collected and obtained from the
institution’s electronic medical records. The following data were
extracted: cohort characteristics, tumor location, intraoperative
outcomes (operative time, estimated blood loss, conversion rate,
complications), postoperative recovery (length of stay (LOS), postoperative
complications), and pathological outcomes (margin status
by frozen section and/or permanent section, tumor size, histologic
grade, lymph nodes harvested). Tumor location and distance from the
SMV were identified using pre-operative CT scans to trace the SMV/
portal vein confluence to the proximal edge of the tumor. The shorter
the distance the more proximal the dissection necessary for resection
and in principle; more challenging the surgery. Postoperative
complications were categorized according to the Dindo–Clavien
classification [16].
Data were stratified into LDP and RDP cohorts for statistical
analyses. The welch two sample t-tests were used to compare mean
age, BMI, length of stay, tumor size and location, console time,
estimated blood loss, and the number of nodes examined. Fisher’s exact
test was used to compare categorical variables including race, gender,
insurance status, ASA score, comorbidities, and post-operative
complications.
Results
Following application of the exclusion criteria, 45 patients,
22 LDPs and 23 RDPs, were included in the study. In regards to
demographics, the two cohorts were not significantly different for
age (mean 59.9 and 63.1 years, respectively), race, gender, BMI (mean
29.6 and 27.7, respectively) and insurance status. The most frequent
American Society of Anesthesiologist’s score (ASA) in the cohorts
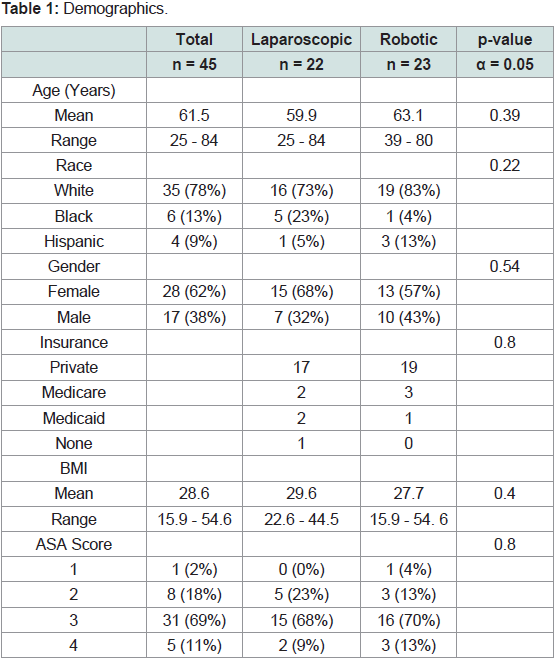
was 3; however, this was also not significantly different (Table 1). No
significant differences in comorbidities, previous cancer, or previous
surgery were observed between the two groups (data not shown).
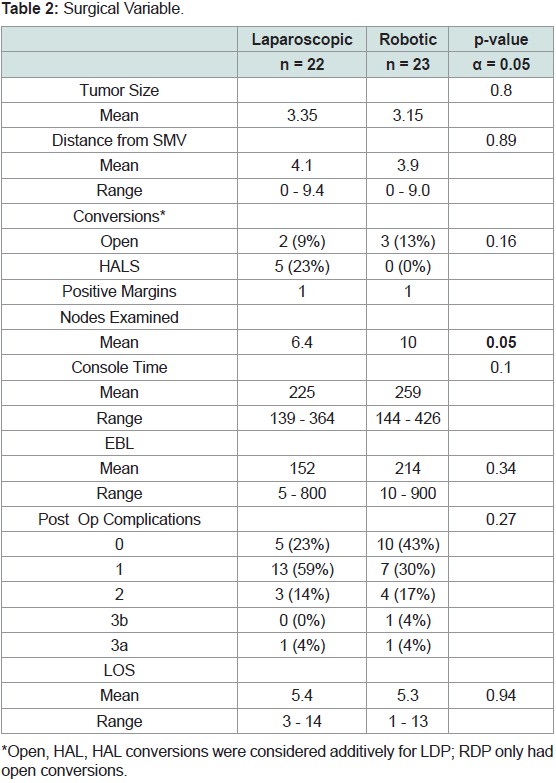
Interestingly, there were no significant differences for tumor size
(LDP: 3.4cm +/- 2.8, RDP: 3.1cm +/- 1.9; p=0.80) or distance from
the SMV (LDP: 4.1cm +/- 3.0, RDP: 3.9 cm+/- 2.9; p = 0.89) between
cohorts (Table 2). When considering conversions, HALS/open were combined for each cohort; overall, while the LDP cohort had more
conversions than the RDP cohort (LDP 32%; RDP 13%), this was not
significantly different (p=0.16; Table 2). Overall, five LDP resections
included a hand assist port (25%), two were converted to open (9%),
while 13% of RDP were converted to open (Table 2).
Additionally, while both cohorts had 5% of resections with
positive margins, LDP harvested less lymph nodes than RDP (mean
6.4 and 10, respectively); this was not significantly different (p=0.09;
Table 2). The estimated blood loss between the cohorts was also not
significantly different (p=0.34) nor was the console time (p=0.10;
Table 2). Post-operative complications and length of stay (mean 5.4
and 5.3 days, respectively) were similar between groups (p=0.27;
p=0.94).
The most common diagnosis for the LDP cohort was a mucinous
cystic neoplasm in five patients (23%). Serous cystic neoplasm was
the most common diagnosis for the RDP cohort, representing seven
patients (30%).
Discussion
As surgical techniques evolve and medical technology advances,
established approaches are replaced with more innovative procedures
that promise better outcomes. Yet before widespread adoption,
the safety, efficacy, and feasibility of these approaches must be
confirmed. Laparoscopic distal pancreatectomy has been shown to
safely improve patient outcomes when compared to a traditional
open approach; however, technical limitations remain [6]. A robotic
approach provides a solution to these technical limitations with its
internal articulation (seven degrees of freedom), 3D perspective, 10X
optics and tremor filtration [10].
The outcomes of LDP and RDP have been compared by numerous
retrospectivestudies. Meta analyses of the literature have concluded
that there is essentially no difference between LDP and RDP regarding
operative time, conversion rate, major morbidity, or post-operative
fistula [14,17]. In our study, we aimed to not only assess these measures
but also the feasibility of adopting a total robotic platform for a senior
surgeon. Anecdotally, adoption of new techniques can be hindered
by a lack of training/experience, comfort with established approaches
and concerns regarding outcomes. To these points, three important
findings were demonstrated in our study. First, within the early
learning phase (20 cases) the senior surgeon was able to match his
laparoscopic outcomes with no increase in operative time, morbidity
or other post-operative complications. Thus, demonstrating that an
“old dog” can safely learn a disruptive technology without sacrificing
outcomes. A comparison of the first 20 RDP compared to our last
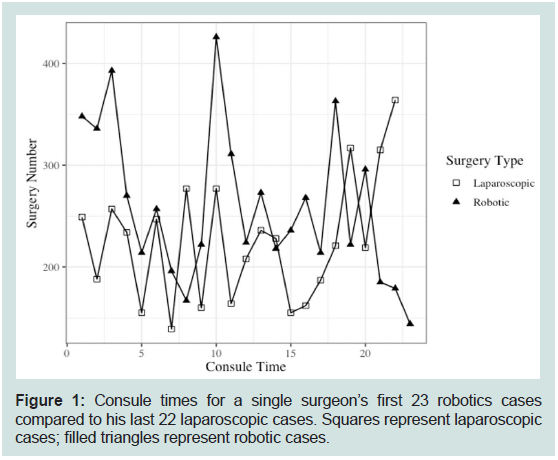
20 LDP highlight this (Figure 1). It is interesting to see that while
there was variability in the RDP times there was also a fair amount in
the LDP, despite these being our established experience (greater than
15 years of LDP resections). Few others have investigated the robotic
learning curve for distal pancreatectomy. Our outcomes mirrored
similar studies. A study comparing a single institution’s first 20
robotic cases with their later 17 cases found no significant difference
in operative time or conversion rate [1]. Another study assessed
the robotic learning curve over a single institution’s first 55 robotic
cases using a cumulative sum method and found that operative time
improved only over the first ten patients [19].
Figure 1: Consule times for a single surgeon’s first 23 robotics cases
compared to his last 22 laparoscopic cases. Squares represent laparoscopic
cases; filled triangles represent robotic cases.
While our study did not elucidate any significant differences
between the LDP and RDP cohorts, once distal pancreatectomies
were done robotically, LDP was no longer a surgical option at
our institution. Thus, surgeon selection bias was eliminated. Like
Daouodi [5], we sought to minimize this bias by evaluating LDP and
RDP cohorts based on time and not on patient or surgeon preference.
Since all DP performed at our institution were laparoscopic before
November 2016 and all DP performed after November 2016 were
robotic, we were able to reduce the effect of treatment selection
bias. Our results add to the growing evidence that RDP can be safely
adopted with proper training and preparation, especially by surgeons
with extensive LDP experience.
Second, while the conversion rates for LDP and RDP were not
significantly different in our study, the overall conversion rate for
LDP was greater than RDP (31% versus 13%), suggesting that this
phenomenon will continue with continued acquisition of skill and
more resection experience. Daouodi described a reduced conversion
rate for RDP and Duran concluded that RDP reduced morbidity
[5,12]. In hindsight this is not surprising when one considers the
improved optics (10x magnification), instrument degrees of freedom
(7 robotic vs 4 laparoscopic) and 3D visualization.
Third, in oncologic outcomes, the number of lymph nodes
resected has become a surrogate for completeness of resection and
improved prognostication [18]. We demonstrated that with RDP
the number of lymph nodes resected was greater than in LDP, 10
vs 6.4 nodes (p=0.09). Again, although we are in the early phase of
our adoption of this approach, we have nonetheless been able to not
only show equivalence but even improvement in some measures of
successful outcomes.
One of the reasons we elected to adopt robotics was the potential
ability to perform a more proximal dissection ( toward the SMV/
portal vein confluence). For LDP, the closer to the SMV/portal vein
the lesion was, the more likely we were to use a HALs approach or
dissect the tail and then make a midline incision (limited open) to
complete the resection at the neck. How to define and assess this
proximal dissection is difficult, outside of anecdotal experience and therefore we present a new variable that is potentially less subjective.
Tumor distance from the SMV/portal vein confluence was evaluated
in an attempt to quantify the anecdotal evidence for robotic surgery
facilitating more proximal dissections. We hypothesized that RDP
might allow surgeons to operate on tumors closer to the SMV/
portal vein confluence, but ultimately found no significant difference
in this metric. A confounding issue in this variable may have been
the resolution of imaging prior to resection regarding the ability to
recreate a 3D-high resolution map of the pancreas and its relationship
to the SMV/portal vein confluence. With more precise measurements,
3D modeling and larger sample size, tumor distance from the SMV/
portal vein confluence might be a valuable variable for future studies.
While the robotic approach has been shown to be a safe, feasible,
and an effective alternative to LDP, its widespread adoption has
likely been hindered by physicians’ comfort with laparoscopic
techniques, the relative lack of data on the RDP learning curve, and
the initial cost of robotics systems [Napoli, 2015]. We present a
single senior surgeon’s transition from laparoscopic to robotic distal
pancreatectomies to demonstrate that “old dogs” can safely learn
“new tricks”. Given that the surgeon had the greatest experience
with LDP and the least experience with RDP highlights and the lack
of difference in outcomes is remarkable and encouraging for other
surgeons.
Conclusion
Our experience suggests that converting to an entirely robotic
approach for distal pancreatectomies is safe, and effective, with
potentially better lymph node dissection and a learning curve that
demonstrates adoption at any level of post-residency training, even if
that is years later. The superiority of the robotic approach over a more
traditional laparoscopic approach continues to be debated; however,
the introduction of a new variable, tumor distance from the SMV/
portal vein confluence, could help quantify the theoretical technical
advantages of robotic distal pancreatectomy. As the technology
continues to evolve and more data are presented, it will be important
to continue these investigations in larger, randomized clinical trials,
especially with regard to long-term outcomes and physician learning
curves.
Acknowledgement
Carter Powell BS, Christine MG Schammel
PhD have no competing interests or financial ties to disclose.
Dr. Trocha is a personal paid consultant for Castle Biosciences,
Johnson and Johnson, and Boston Scientific; this study was not
affected by any of these companies.
No funds, grants or other support was received in the execution
of the study or preparation of the manuscript.
The datasets generated during and/or analyzed during the
current study are not publicly available, but are available from the
corresponding author on reasonable request and the procurement of
a data sharing agreement with Prisma Health Upstate.
Ethics:
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the
IRB of Health Sciences SC who determined that our study did not
need ethical approval. An IRB official waiver of ethical approval
was granted from the IRB of Health Sciences SC and Prisma Health
Upstate.