Journal of Toxins
Download PDF
Research Article
*Address for Correspondence: Akintunde J.K, Drug Metabolism and Molecular Environmental Toxicology Research Laboratory, Biochemistry unit, Department of Biosciences and Biotechnology, College of Pure and Applied Sciences, Kwara State University, Malete, P.M.B 1530, Ilorin, Nigeria, Fax: +23408064156056; E-mail: akintundejacob@yahoo.com
Citation: Ajiboye JA, Akintunde JK, Oladejo OS, Sabiu SA. Chemoprevention of Silymarin and Vitamin C on Isoniazid-Induced Hepatotoxicity in Experimental Rat Model. J Toxins. 2015;2(1): 3.
Copyright © 2015 Akintunde et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 2, Issue: 1
Submission: 23 June 2015 | Accepted: 29 July 2015 | Published: 03 August 2015
Chemoprevention of Silymarin and Vitamin C on Isoniazid-Induced Hepatotoxicity in Experimental Rat Model
Ajiboye J.A1, Akintunde J.K2*, Oladejo O.S1, and Sabiu S.A2
- 1Department of Chemical Sciences, Biochemistry unit, College of Natural and Applied Sciences, The Bells University of Technology Ota, Ogun State, Nigeria
- 2Drug Metabolism and Molecular Environmental Toxicology Research Laboratory, Biochemistry unit, Department of Biosciences and Biotechnology, College of Pure and Applied Sciences, Kwara State University, Malete, P.M.B 1530, Ilorin, Nigeria
*Address for Correspondence: Akintunde J.K, Drug Metabolism and Molecular Environmental Toxicology Research Laboratory, Biochemistry unit, Department of Biosciences and Biotechnology, College of Pure and Applied Sciences, Kwara State University, Malete, P.M.B 1530, Ilorin, Nigeria, Fax: +23408064156056; E-mail: akintundejacob@yahoo.com
Citation: Ajiboye JA, Akintunde JK, Oladejo OS, Sabiu SA. Chemoprevention of Silymarin and Vitamin C on Isoniazid-Induced Hepatotoxicity in Experimental Rat Model. J Toxins. 2015;2(1): 3.
Copyright © 2015 Akintunde et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | ISSN: 2328-1723 | Volume: 2, Issue: 1
Submission: 23 June 2015 | Accepted: 29 July 2015 | Published: 03 August 2015
Abstract
Isoniazid, anti-mycobacterial agent employed clinically in the treatment of bacterial infections (tuberculosis), is known to cause a number of biochemical dysfunctions and suspected to induce hepatic damage to animals and humans. However, co-administration of antioxidants to ameliorate the possible ill effect of anti-tuberculosis medications should be advocated. The present study therefore evaluates its toxicity in liver cells of male rats and the chemo-preventive effect of Silymarin (SIL) and Vitamin C. Administration of Isoniazid caused a significant (p< 0.05) increase in the activities of plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST). While SIL and Vitamin C significantly reversed the toxicity effect induced by antibiotic drug. Collectively, the results suggest that therapeutic dose of Isoniazid elicits hepatotoxicity in male rats. The chemo-protection effects of SIL and Vitamin C during Isoniazid treatment suggest their clinical applications in hepatic damage and may serve as adjunct in tuberculosis therapy.Keywords
Isonicotinic acid hydrazine; Hepatotoxicity; Tuberculosis; Chemoprevention; Sylimarin; Vitamin CIntroduction
Isoniazid or isonicotinic acid hydrazine (INH) is a potent antimycobacterial agent which acts by inhibition of lipid and DNA synthesis of Mycobacterium tuberculosis, thus inhibiting its cell wall synthesis [1]. INH was introduced into clinical practice in 1952 and contributed greatly to the subsequent dramatic decrease in morbidity and mortality caused by tuberculosis. At the time of introduction, Isoniazid was found to have few side effects, excellent compliance rate and highly efficacious. It rapidly became the mainstay of antituberculosis therapy. However, cases of severe hepatotoxicity, skin reactions, gastrointestinal and neurological disorders due to isoniazid soon appeared and clearly defined its toxic potentials [2]. These ill effects of INH have been well researched and induction of oxidative stress via free radicals formation and reactive oxygen species (ROS) were opined as possible mechanisms [3]. Following these dual attributes, INH usage was then either restricted or modified for administration alongside antioxidants to effectively ameliorate the insults of ROS induced cellular damage.Silymarin is a flavonoid extracted from the seed of Silybum Marianum (milk thistle plant). Silybum marianum is a member of the aster family (Asteraceae or Compositae) which encompasses daisies and thistles [4]. Silybin (silibinin), silychristin, and silydinin have been identified as its major active ingredients [5]. This makes it as a common herbal therapy particularly for treating liver diseases partly due to its antioxidant activity [6]. It has membrane-stabilizing and antioxidant activity, promotes hepatocyte regeneration, reduces inflammatory reaction, and inhibits fibrogenesis [7-9].
Vitamin C is a six-carbon compound structurally related to glucose, found in citrus, soft fruits and leafy green vegetables [10]. It is hydrophilic in nature and had been implicated as free radical scavenger in extracellular fluids and protecting bio-membranes from peroxide damage [11-19]. Recently, since the liver plays a major role in the metabolism of drugs and consequently the primary target of most toxic responses. Research has largely concentrated on liver functions. However, co-administration of antioxidants to ameliorate the possible ill effect of anti-tuberculosis medications should be advocated. Hence, the present study seeks to substantiate the latter fact by exploring the antioxidant modulatory effects of silymarin and vitamin C on INH induced hepatotoxicity in experimental animal models.
Materials and Methods
Chemicals and reagentsAssay kits for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were products of Randox Laboratories limited, United Kingdom. Distilled water used was glass distilled. Tablets of isoniazid and silymarin were procured from Sigma-Aldrich Chemicals Company (St. Louis, Mo, USA). Vitamin C was purchased from Emzor Pharmaceutical Industries limited, Lagos, Nigeria. Other chemicals and reagents were all of analytical grade.
Experimental protocol
Wistar strain albino rats having the weight of 180.00±2.33 gwere purchased from the animal house of the University of Ibadan, Nigeria. They were kept in cages in a well ventilated room maintained at a temperature of 25±2 °C with a 12 h light-dark cycle for ten days to acclimatize, and were allowed free access to food and water ad libitum. The protocol conforms to the guidelines of the National Institute of Health for laboratory animal care and use [20]. Twenty five albino rats were randomized into five groups of five rats each. Group I serves as the control, animals in Groups II, III, IV and V received 28.6 mg/kg body weight of Isoniazid (INH) per day which represents the therapeutic dose in humans. Group III was co-treated with SIL at therapeutic dose of 200 mg/kg body weight per day. Group IV was co-treated with Vitamin C at dose of 50 mg/kg body weight per day. Lastly, group V was co-treated with SIL and Vitamin C at doses of 200 and 50 mg/kg body weight per day respectively, and the experiment last for 14 days. Food and water were administered ad libitum throughout the period of the experiment. After the last administration, the animals were fasted overnight and were humanely sacrificed by cardiac puncture. Blood sample was collected in heparinized bottles, centrifuged at 3000 rpm for 15 min. The resulting plasma was carefully aspirated with a Pasteur’s pipette into sample bottles for liver enzymes assay.
Liver enzymes assay
Plasma of hepatic alanine aminotransaminase (ALT) and hepatic aspartate aminotransaminase (AST) were estimated by the method of Reitman and Frankel [21].
Statistical analysis
All data were subjected to one-way analysis of variance (ANOVA) using SPSS software package for windows (Version 16) and expressed as mean ± standard deviation (SD) (n=5). Significant difference between the treatment means was determined at 5% confidence level using Duncan’s Multiple Range Test.
Results
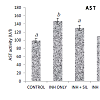
The present investigation revealed the chemo-protection effect of silymarin and vitamin C on isoniazid-induced hepatotoxicty in male rats. As observed (Figure 1), the activities of plasma AST was significantly (p< 0.05) increased in isoniazid induced by 4762% when compared with the corresponding control. While the observed high activities of plasma AST was significantly (p< 0.05) reversed in the animals co-treated with INH + SIL, INH + Vit C and INH + SIL+ Vit C by 3082%, 1096% and 404% respectively (as shown in Figure 1). Similarly, it was observed (Figure 2) that the activities of plasma ALT was significantly (p< 0.05) increased in isoniazid induced by 4748% when compared with the corresponding control. The observed elevations were however significantly attenuated (P < 0.05) in the rats co-treated with INH + SIL, INH + Vit C and INH + SIL+ Vit C by 3175%, 1935% and 235% respectively (as shown in Figure 2). In addition, Vit C has a higher reversal effect than silymarin in Isoniazid induced hepatotoxicity. Collectively, as shown in this study, coadministration of Vitamin C and silymarin exhibited high efficacy in treating liver problems than when they are singly co-administered.Discussion
The liver is susceptible to injury by xenobiotics such as drugs. One of the most serious and frequent adverse effects of anti-tuberculosis drugs is hepatotoxicity [2]. For effective administration and compliance however, co-prescription with antioxidants have been advocated over the years. This is the focus of the present investigation.Antioxidants elicit protective potentials by interacting with biomolecules at cellular and molecular levels to induce cytoprotective enzymes or inhibits those involve in carcinogen activation. Alteration in activity of liver marker enzymes like alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) suggests a possible damage to the hepatocyte membrane and thus a compromised integrity and permeability of the membrane [22]. The significant increase in plasma activity of AST and ALT following administration of INH in the treated animals may be attributed to generation of free radicals. This might trigger chains of reactions resulting in liver damage. It also suggests possible leakage of the enzymes resulting from susceptibility of the hepatocyte membrane to adverse influence of INH which could have consequential effect on the metabolism and regulation of other enzymes in the liver [23]. This agrees with the findings of Snodgross et al., Karthikryan and Adebayo AJ et al. where INH was reported to have caused hepatic damage in experimental animals manifested by increased activities of liver marker enzymes in the serum [3,24,25]. Conversely, the significant reduction in enzyme activities of rats co-administered with either silymarin or vitamin C and both suggests that both molecules were able to ameliorate the deleterious effects of INH on the rat’s hepatocytes.
Both silymarin and vitamin C have been reported to have excellent antioxidant potentials against oxidative stress induced cellular damage [6,15,19,26,27]. Thus, the effects elicited by co-administration of the two molecules could possibly be attributed to their electron-donating capacities to form stable products and subsequently, terminating free radical chain reactions. The membrane-stabilizing and antioxidant activity which promotes hepatocyte regeneration as reported by Shalan et al. for silymarin and vitamin C might also be linked to the reduction in the enzymes activities of rats co-administered [28].
Conclusion
The present study reveals that administration of therapeutic dose of isoniazid to male rats induced hepatotoxicity by increasing the key markers of liver damage- AST and ALT. SIL and Vitamin C exhibited similarities in their capability to mitigate the toxic responses of isoniazid, which suggests that the adverse effects of isoniazid on the liver are due to leakage of liver enzymes from mitochondrial matrix of hepatic cells. However, co-administration of Vitamin C and silymarin exhibited high efficacy in treating liver problems than when they are singly co-administered.References
- Hussain Z, Kar P, Husain SA (2003) Antituberculosis drug induced hepatitis: risk factors, prevention and management. Indian J Exp Biol 41: 1226-1232.
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C (2003) Tuberculosis. Lancet 362: 887-899.
- Adebayo AJ, Kehinde AJ, Adetokunbo OA, Olamide AE, Oluwatosin A (2012) Influence of isoniazid treatment on microsomal lipid peroxidation and antioxidant defense systems of rats. J Drug Metab Toxicol 3: 120.
- Saller R, Melzer J, Reichling J, Brignoli R, Meier R (2007) An updated systematic review of the pharmacology of silymarin. Forsch Komplementmed 14: 70-80.
- Rui YC (1991) Advances in pharmacological studies of silymarin. Mem Inst Oswaldo Cruz 86: 79-85.
- Tong S, Chu C, Wei Y, Wang L, Gao X, et al. (2011) Preparation and effects of 2,3-dehydrosilymarin, a promising and potent antioxidant and free radical scavenger. J Pharm Pharmacol 63: 238-244.
- Valenzuela A, Garrido A (1994) Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res 27: 105-112.
- Shaker E, Mahmoud H, Mnaa S (2010) Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol 48: 803-806.
- Feher J, Lengyel G (2012) Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol 13: 210-217.
- Stangeland T, Remberg SF, Lye KA (2009) Total antioxidant activity in 35 Ugandan fruits and vegetables. Food Chem 113: 85-91.
- McDowell LR (1989) Vitamin C. In: Vitamins in animal nutrition: Comparative aspects to human nutrition. Academic Press London, pp. 93-131.
- Bendich A (1990) Antioxidant micronutrients and immune responses. In Bendich and R. K. Chandra, Eds., Micro- nutrients and Immune Functions, Academy Sciences, New York, 175.
- Sies H, Stahl W, Sundquist AR (1992) Antioxidant functions of vitamins. Vitamin E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci 669: 7-20.
- Burtis CA, Ashwood ER (1994) Tietz textbook of clinical chemistry, 2nd edition, WB Saunders Co., Philedelphia, pp. 1275-1512.
- Sminorff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35: 291- 341.
- Cazenave J, Bistoni Mde L, Pesce SF, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76: 1-12.
- Xavier SM, Barbosa CO, Barros DO, Silva RF, Oliveria AA, et al. (2007) Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci Lett 420: 76-79.
- Zhang X, Yang F, Zhang X, Xu Y, Liao T, et al. (2008) Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat Toxicol 86: 4-11.
- Ergul Y, Erkan T, Uzun H, Genc H, Altug T, et al. (2010) Effect of vitamin C on oxidative liver injury due to isoniazid in rats. Pediatr Int 52: 69-74.
- NIH (1985) Care and use of laboratory animals. National Institute of Health Publication.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 56-63.
- Sabiu S, Wudil AM, Sunmonu TO (2014) Combined administration of Telfairia occidentalis and Vernonia amygdalina leaf powders ameliorates garlic-induced hepatotoxicity in Wstar rats. Pharmacologia 5: 191-198.
- Sunmonu TO, Sabiu S, Ugbaja RN, Akinloye AO (2014) Toxicological effects of aqueous and ethanolic root extracts of Strophanthus hispidus on kidney and liver function indices of Wista rats (In press).
- Snodgross W, Potter W, Timbrell JA, Jollow DI (1974) Possible mechanism of isoniazid related hepatic injury. Clin Res 22: 323-326.
- Karthikryan S (2004) Hepatotoxicity of isoniazid: A study in the activity of marker enzymes of liver toxicity in serum and liver tissue of rabbits. Indian J Pharmacol 36: 247-249.
- Tomankova K, Kolarova H, Kolar P, Kejlova K, Jirova D (2009) Study of cytotoxic effect of photodynamically and sonodynamically activated sensitizers in vitro. Toxicol In Vitro 23: 1465-1471.
- Mongi S, Mahfoud M, Amel B, Kamel J, Abdelfattah el F (2011) Protective effects of vitamin C against haematological and biochemical toxicity induced by deltamethrin in male Wistar rats. Ecotoxicol Environ Saf 74: 1765-1769.
- Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A (2005) Amelioration of lead toxicity on rat liver with Vitamin C and silymarin supplements. Toxicology 206: 1-15.