ZAP70-SCID syndrome is a hereditary genetic disorder that
damages the immune system. SCID-dependent ZAP70 syndrome is
one of the forms of severe immunosuppression that is caused by several
genetic causes [
1]. Severe Combined Immunodeficiency (SCID)
comprises a group of rare monogenic primary immunodeficiency
disorders characterized by a lack of functional peripheral T
lymphocytes resulting in early-onset severe respiratory infections
and failure to thrive. They are classified according to immunological
phenotype into SCID with absence of T cells but presence of B cells
(T-, B+, SCID) or SCID with absence of both (T-,B- SCID) (see these
terms). Both of these groups include several forms, with or without
natural killer (NK) cells [
1].
Clinical symptoms of ZAP70 related SCID- syndrome (ZAP70- SCID):
Children with SCID have virtually no immune system against
bacterial, viral, and fungal infections. They are susceptible to
frequent and persistent infections that can be very serious and life
threatening. Most microorganisms that cause infection in people
with this disorder are described as opportunistic microorganisms,
because they usually do not cause disease in healthy people. Infants
with ZAP70-SCID typically have pneumonia, chronic diarrhea, and
extensive rash (rash). They also grow much slower than healthy
children. If the immune system of these children does not have the
ability to fight infection, they usually die one or two years after the onset of infections. Most people with ZAP70-SCID syndrome are
diagnosed in the first 6 months of life [
2].
Etiology of ZAP70 related SCID- syndrome (ZAP70-SCID):
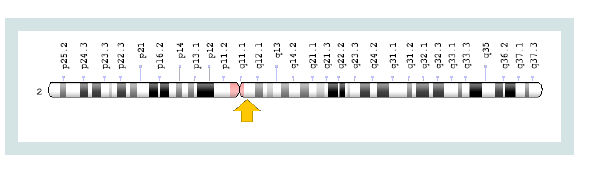
ZAP70-SCID syndrome is caused by the mutation of the ZAP70
gene, which is based on the long arm of chromosome number 2 as
2q11.2. The gene provides instructions for the synthesis of a protein
called zeta chain protein kinase. This protein is part of the signaling
pathway that creates and activates immune cells called T cells. T cells
detect external material and protect the body from infection (
Figure 1) [
3].
The ZAP70 gene is important for the development and function
of several T cell types. These include cytotoxic T cells (CD8+ T cells)
whose function involves destroying virus-infected cells. The ZAP70
gene is also involved in the activation of T helper cells (CD4+ T cells).
These cells directly contribute to the immune system by influencing
the activities of other immune cells [
4].
The mutation in the ZAP70 gene blocks the production of Zetalinked
protein kinase, or leads to a protein that is unstable and cannot
function. Loss of zeta-functional protein kinase results in the absence
of CD8+ T cells and excessive inactivation of CD4+ T cells. The
deficiency of active T cells causes those who have ZAP70-dependent
SCID (ZAP70-SCID) to become more susceptible to infection [
5].
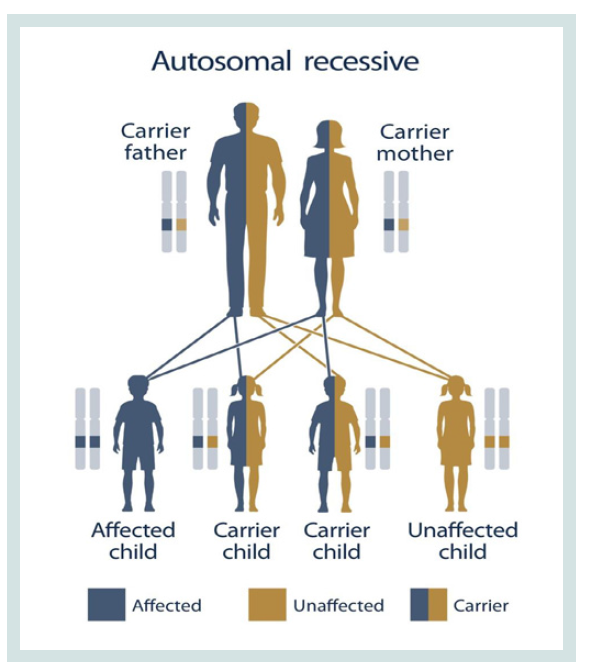
ZAP70-SCID syndrome follows an autosomal recessive hereditary
pattern. Therefore, in order to produce this syndrome, two copies of
the mutated gene ZAP70 (one parent and one mother) are needed,
and the chance of having a child with this syndrome in an autosomal recessive state is 25% for each pregnancy [
6].
The prevalence of ZAP70 related SCID- syndrome (ZAP70-SCID):
ZAP70-SCID syndrome is a rare genetic disorder that has been
reported in about 20 cases of this syndrome from around the world in
medical literature. The incidence of SCID disorder is approximately 1
in 50,000 people [
7]. Overall incidence is estimated at about 1/50,000
live births, with regional differences and higher incidences among
populations with a higher consanguinity rate. The disease affects
more males because of the X-linked variant (SCID T-B+ due to
gamma chain deficiency; see this term) that represents about 30% of
SCID cases in Western countries.
Detection of ZAP70 related SCID- syndrome (ZAP70-SCID):
The ZAP70-SCID syndrome is diagnosed based on the clinical
and clinical findings of the patients and some pathological tests. The
most accurate method for detecting this syndrome is the molecular
genetic testing of the ZAP70 gene to investigate the presence of
possible mutations [
7]. Early diagnosis, before the infant has had
a chance to develop any infections, is very valuable since bone
marrow transplants given in the first three months of life have a 94%
success rate. In fact, screening newborns to detect SCID soon after
birth has been made possible because of recent scientific advances.
Approximately half of the babies born in the U.S. are now being
screened for SCID [
7]. The Genetic Testing Registry (GTR) provides
information about the genetic tests for this condition. The intended
audience for the GTR is health care providers and researchers.
Patients and consumers with specific questions about a genetic test
should contact a health care provider or a genetics professional [
7].
An Action (ACT) sheet is available for this condition that describes
the short-term actions a health professional should follow when an
infant has a positive newborn screening result. ACT sheets were
developed by experts in collaboration with the American college of
medical genetics. Without treatment SCID usually results in severe
infection and death in children by age of 2. When performed from
an HLA-identical sibling, and in the first few months of life, HSCT
can result in a greater than 90% long-term survival rate (
Figure 2) [
7].
Therapeutic routes of ZAP70 related SCID- syndrome (ZAP70- SCID):
The ZAP70-SCID syndrome treatment and management strategy
is symptomatic and supportive. Treatment may be done by a team of
experts, including immunologist, dietitian, pulmonary specialist and
other health care professionals. There is no standard treatment for
this syndrome, and all clinical interventions are designed to reduce
the suffering of the sufferers. Genetic counseling is also a special place
for all parents who want a healthy baby [
7]. The child will be admitted
to a room or an area with ‘filtered air’ (to remove germs). He or she
will be confined to this room and will not be able to mix with other
children or go to the ward playroom. Parents are able to stay with
their child and will be encouraged to continue to feed, care for and
play with him or her as much as they want. Visitors will be kept to a
minimum, and no one who has an infection will be allowed to visit.
Parents will be told about the ways that they can avoid passing on
infection, such as washing their hands thoroughly (
Figure 3) [
7].
Further blood tests will be performed to confirm the diagnosis
and type of SCID. More specialized tests will subsequently be carried
out to determine the precise genetic abnormality. Other investigations
will also be necessary to identify any undetected infection, including
chest x-rays, scans and tests on samples of blood, urine, faeces and
mucus from the throat [
7].