Journal of Veterinary Science & Medicine
Download PDF
Research Article
Pharmacokinetics of Artemisinin in Broiler Chickens after Intravenous and Oral Administrations
Ali Rassouli*, Hossein Ali Arab, Ismael Imani and Gholam Reza Shams
- Department of Pharmacology, Faculty of Veterianry Medicine, University of Tehran, Tehran, Iran
*Address for Correspondence:Ali Rassouli, Department of Pharmacology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran, Tel: +9821-61117086; Fax: +9821-66933222; E-mail: arasooli@ut.ac.ir
Citation: Rassouli A, Arab HA, Imani I, Shams GR. Pharmacokinetics of Artemisinin in Broiler Chickens after Intravenous and Oral Administrations. J Veter Sci Med. 2016;4(2): 4.
Copyright: © Rassouli A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 4, Issue: 2
Submission: 26 September, 2016| Accepted: 20 October, 2016 | Published: 31 October, 2016
Abstract
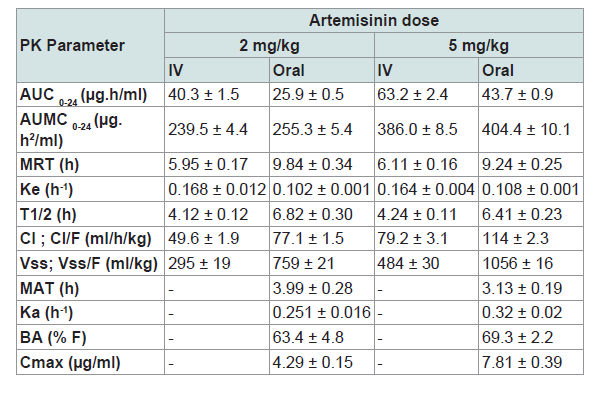
Artemisinin (ART), the active ingredient of Artemisia spp. is a promising anticoccidial agent. Since there is no documented information on pharmacokinetics (PK) of this herbal drug in poultry, the present study was carried out to explore PK parameters of ART in broiler chickens. Sixty-four healthy broiler chicks of 21 days old were randomly divided into 4 equal groups. In groups 1 and 2, each bird received a single dose of ART at 2 mg/kg by oral (PO) and intravenous (IV) administrations, respectively. But each bird in groups 3 and 4 was given 5 mg/kg ART by PO and IV, respectively. Blood samples were taken at different pre-determined time-points. The serum concentrations of ART were measured by using a high performance liquid chromatographic (HPLC) method. PK parameters of ART (including maximum concentration, Cmax, time to reach maximum concentration, Tmax, area under the drug concentration-time curve, AUC, mean residence time, MRT, volume of distribution at steady state, Vss, and bioavailability, BA) were calculated through noncompartmental analysis. The Cmax values for 2 and 5 mg/kg were 4.29 ± 0.15 and 7.81 ± 0.39 µg/ml; and the BA values (%) were 63.4 ± 4.8 and 69.3 ± 2.2, respectively. The Vss values after IV administration in groups 2 and 4 were 295 ± 19 and 484 ± 30 ml/kg, respectively. The relatively high BA and a low Vss for ART in the present study suggest that there is a species difference in PK of this compound in poultry.
Keywords
Artemisinin; Coccidiosis; Pharmacokinetics; Bioavailability; Poultry
Introduction
Coccidiosis is one of the important parasitic diseases all over the world that causes a large economic loss in poultry industry. The control of coccidiosis is mainly based on the use of anticoccidial agents in feed. Owing to the ongoing drug resistance and residue problems in poultry production, there are increasing interests toward using alternative and safe compounds for control of coccidiosis. In recent years, a number of studies have been directed toward the anticoccidial activity of natural products such as essential oils and plant extracts [1,2].
Artemisinin (ART) is the active ingredient of Artemisia spp. with well-known antimalarial effects [3]. It has been used for centuries to treat malaria, gastrointestinal helminthosis, hemorrhoid, skin rashes and some other diseases especially in oriental medicine [4]. For the first time, in 1972, ART or qinghaosu was isolated from the leaves of the plant, Aretemisia annua, by Chinese researchers, as a sesquiterpene lactone. Thereafter, a number of its derivatives such as artesunate, artmether, arteether have been synthesized to improve its bioavailability in humans [3]. ART has got a lot of interest in medical settings because of its rapid antimalarial action on drug-resistant forms of Plasmodium falciparum [5]. It has also demonstrated antimicrobial, antiviral, anticancer, anthelmintic as well as antiangiogenic and immunomodulatory effects [3,5-8]. In the field of veterinary medicine, ART has attracted the attention of a great number of researchers to explore its activity against Eimeria spp.the causative agents of avian coccidiosis [9-11]. Furthermore, the inhibitory effects of ART against other protozoan parasites such as Toxoplasma gondii, Neosporacaninum, Theileriaequi or Leishmania donovani have been shown at least in a number of in vitro studies [11-13].
The chemotherapeutic activity of ART and its semi-synthetic analogs is thought to be through the reactivity of the endoperoxide bridge, the common structural feature of ART and all of its active derivatives [3]. ART and its analogs are generally regarded as low toxic agents since a lot of studies in humans have demonstrated its excellent safety and tolerability as well as shown in some animal species such as rats, dogs and chickens [3,4,14,15].
Although, there are numerous studies on PK of ART and its derivatives alone or in combination with other antimalarial agents in humans, as well as a few related studies in rats and dogs [3], there are few, if any, PK studies on ART in poultry. Therefore, the present study aimed to explore PK parameters of ART following IV and PO administrations in broiler chickens.
Materials and Methods
Animals and drug administration
Sixty-four healthy day-old broiler chicks (Ross 308) were purchased from a local poultry farm. They were kept in metal cages for three weeks with free access to conventional feed and water as well as 12 h light/darkness cycles. The birds were randomly divided into four groups of 16 birds one week before drug administration. Animals were kept fast for 12 h before experiment and 4 h following the drug dosing, however, water was accessible to birds except 2 h before and 2 h after drug administration. Pure ART (>90%) as colorless crystals were purchased from Sichuan Arts and Crafts Import & Export Corporation, China. ART solutions were prepared by dissolving pure ART in dimethylsulphoxide (DMSO) and then diluted by adding water. On day 21, in a parallel design, birds were treated with different doses and routes of administration. In groups 1 and 2, each bird received 2 mg/kg ART by PO and IV administrations, respectively. But each bird in groups 3 and 4, was given 5 mg/kg ART by PO and by IV, respectively.
This study was a randomized, parallel group, double blind, placebo controlled trial for assessing the safety and efficacy of cosmetic product Romantaque Lotion in healthy human volunteers according to IS 4011:1997/2009 Guidelines for three hair growth cycles. This clinical study included two protocols to evaluate safety and efficacy of the test material, Draize test- (RMTK 01) and Use Test- (RMTK 02). The study was carried out between 15 March 2013 and 13 February 2014 at National Toxicology Centre, Pune. The study protocol was approved by an Independent Ethics Committee, Informed written consent were obtained from all the volunteers before enrollment for study. This study has been registered with Clinical Trials Registry of India (CTRI) - CTRI/2015/02/005534.
This study was approved by the Reviewing Board of Ethics Committee, Faculty of Veterinary Medicine, University of Tehran.
Blood sampling and HPLC analysis
Blood samples were taken before drug administration from all 16 broiler chickens in each experimental group and at 1, 2, 4, 8, 12 and 24 h after drug administration from 8 birds in each time points alternatively. The sera of blood samples were collected within 2 h and kept at -20 ºC until analysis. ART in serum samples was extracted by liquid-liquid extraction and measured by an HPLC method with precolumn alkaline derivatization [9,16].
The HPLC system used to measure ART in sera briefly consisted of a C18 column (5 μm; 300* 4.6 mm) and a mobile phase (phosphate buffer, pH=7.9 (60%): Methanol (40%)), running isocratically with a flow rate of 1 ml/min, and a UV detector set at 260 nm. Standard calibration curve was depicted and used for measurement of ART concentrations in serum samples.
PK analysis
ART concentration- time curves of each group depicted using Excel 2013. Cmax and Tmax values were obtained directly from the curves drawn for each experimental group. Other PK parameters of drug calculated through non-compartmental analysis (NCA) and using corresponding formula. The area under the plasma concentration-time curve (AUCo-t) was calculated using the linear trapezoidal rule to the last point. The mean residence time (MRT) was obtained by dividing the area under the first moment-time curve (AUMC0-t) by the area under the curve (AUC0-t). Total oral body clearance (Cl or CL/F) was calculated as dose/AUC0-t. Systemic BA (orally absorbed fraction, F) was calculated as F = AUCPO/AUCIV. Remaining PK parameters including volume of distribution at steady state, Vss, or Vss/F, and mean absorption time, MAT, as well as elimination rate constant, Ke, absorption rate constant, Ka, and elimination half-life, t½, were also calculated using the equations of NCA [17]. Data were expressed as the mean ± standard deviation in this study.
Results
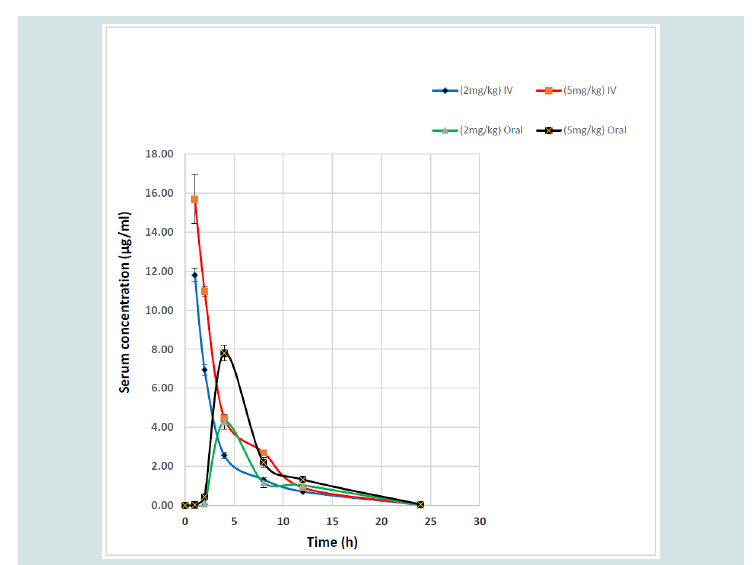
Serum concentration-time curves of ART following IV and oral administrations at 2 and 5 mg/kg in broiler chickens were shown in Figure 1. There were similar patterns of fluctuations in serum concentrations of ART following two doses in oral administration as well as IV dosing. Typical blood level changes following IV administrations were found and peak serum concentrations after oral administration were obtained at 4 h post-dosing for both ART dosages. PK parameters of ART after IV and oral administrations in broiler chickens were also seen in Table 1.
Figure 1: Serum concentration-time profiles of artemisinin after intravenous (IV) and oral administrations at 2 and 5 mg/kg in broiler chickens. Data represent the mean ± SD (n=8).
Discussion
The PK analysis of ART in this parallel study using two doses by two routes of administration showed that ART following a single dose is largely bioavailable in broiler chickens. Moreover, the lower values of volume of distribution obtained in the present study suggest a species variation when compared to those of humans and a few animal species that have already been studied [3].
The results of the present study showed that the mean value ofCmax following oral dosing of 2 mg/kg ART in broiler chickens was 4.29 ± 0.15 µg/ml. It is about 20 or 10 times more than those reported in adult humans receiving 250 mg ART as a low dose (205 ng/ml) or 500 mg as a medium dose (450 ng/ml), respectively [17]. Duc et al. reported a Cmax value of 391 ± 147 ng/ml after a single dose of 500 mg ART in healthy Vietnamese subjects [18]. A Cmax value of 587.4 ± 385 ng/ml was also reported for ART following oral administration of 500 mg in adult patients with symptomatic malaria in Tanzania [19]. In another study, a Cmax value of 260 ng/ml was obtained after oral dosing of ART in 10 human subjects at 5.3 mg/kg [3]. One of the interesting finding in the present study was the less inter-individual variability among serum ART levels in broiler chicken than those reported in humans.
In addition, the values of PK parameters of ART reported by some studies in a few animal species are different. In goats, a Cmax value of 0.7 ± 0.02 µg/ml for dihydro-ART at 12 h after oral administration of ART at 23 mg/kg was reported by Ferreira and Gonzalez, [20]. They suggested that the low oral BA for ART in goats was due to higher levels of unabsorbed ART in feces (2.41 µg/g) at 24 h post dosing. Li reported that the Cmax value of ART in dogs following a single oral administration at 1000 mg/dog was 29.1 ± 9.9 ng/mL [21]. Although this value is less than those found in humans but much lower than that of broiler chickens shown in the present study. On the other hand, They also found that ART plasma concentrations on the 5th day following daily oral administration of 1000 mg/dog was significantly lower than that of the 1st day so that they were non- detectable [21].
There is also much difference between the values of apparent volume of distribution (Vd) in broiler chickens in comparison to humans. In oral dosing of ART at 2 and 5 mg/kg in the present study, the mean Vss/F values were 759 and 1056 ml/kg, respectively. However, in humans these values were 38.4 and 35.5 L/kg at oral dose of 250 and 500 mg ART, respectively [17]. A Vss value of 19.4 ± 6.9 L/ kg after a single dose of 500 mg ART in healthy Vietnamese subjects was also reported by Duc et al. [18]. In a crossover study conducted in 15 healthy male Vietnamese volunteers under fasting conditions, Hien et al. used a single oral dose of 160 or 500 mg of ART and reported Vss/F values of 1420 ± 490 L and 1560 ± 860 L, respectively [15]. These large Vd values for ART have been reported in humans despite a relatively high plasma protein binding of 80-85% [22].
The values of Vd reported by some researchers in animals are also different. Li reported that the mean Vss value of ART in dogs following a single IV dosing at 60 mg per dog was 26.0 ± 10.6 L [21]. In another study conducted by China Coop. Res. Group (1982) in rats at 150 mg/kg ART, i.v., the Vss value was 4.1 L/kg which is much higher than our data in broilers (484 ml/kg) following 5 mg/kg ART, i.v. [3].
In the present study, the BA value for a single oral dosing of ART in broilers was about 65%. But according to the study of Li, the BA value of ART in dogs after a single PO dosing was very low [21]. The oral BA of ART in goats reported by Ferreira and Gonzalez, was also poor. They found that most of the administrated doses in goats were unabsorbed and excreted via feces possibly due to adhesion to GI content [20].
Since there is no report on IV administration of ART in humans to calculate absolute BA, a relative BA of ART in humans based on AUC of PO/IM dosing has been reported as 32% [22]. Despite a presumed high absorption of ART through the PO route, it is believed that the BA to be low due to a significant first-pass extraction. A possibly saturable first-pass metabolism and decreased plasma concentration of ART upon repeated administration have been suggested [22]. There is evidence that relative oral BA of ART in humans greatly decreases following multiple doses, most possibly due to an induction of its metabolism in liver rather than the reduction of absorption [3]. Even though this auto induction in metabolism has been documented by some studies, low blood concentration of parent compound do not seem to influence its therapeutic efficacy due to production of active primary metabolite (dihydro-ART) [3,22].
In the present study, the elimination half-lives of ART in both routes of administration did not change by increasing the dose. In addition, the similar profiles of ART serum concentrations after two doses in both routes of administration indicates that PK processes including elimination in both doses worked in similar ways. This finding differs from that of Ashton et al. who reported increased in half-lives in healthy Vietnamese adults by increasing the ART doses [17]. Meanwhile, according to the findings of Gordi, there was no significant difference in the elimination half-lives between rectal and PO administration of ART [22]. In addition, we found that the MRT value of ART with oral dosing of 5 mg/kg in broilers (9.24 ± 0.25 h) was about two times more than those reported by Ashton et al. in humans (4.49 ± 0.60 h) who received a single oral dose of 500 mg [17].
There are a great number of reports on the low toxicity and good efficacies of this herbal drug and its derivatives as promising chemotherapeutic agents against diverse parasitic and microbial pathogens in humans and animals as well as cancer cell lines [3-6,12-14,22]. Due to the evidence of species differences as an important finding of the present study, more researches and understanding on PK and pharmacodynamics of ART and its analogs in animals are warranted.
In conclusion, this study showed a relatively high BA and a very low Vss for ART in broiler chickens. It is suggested that there is a species difference in the extent of absorption and volume of distribution of ART after a single dose in poultry.
References
- Tewari AK, Maharana BR (2011) Control of poultry coccidiosis: changing trends. J Parasit Dis 35: 10-17.
- Abbas RZ, Colwell DD, Gilleard J (2012) Botanicals: an alternative approach for the control of avian coccidiosis. Worlds Poultry Sci J 68: 203-215.
- World Health Organization (2006) Artemisinin derivatives : summary of nonclinical safety data. Nonclinical overview for artemisinin derivatives. World Health Organization, Geneva, pp. 2-4.
- Arab HA, Mardjanmehr SH, Shahbazfar A, Rassouli A, Abdollahi M, et al. (2009) Toxicopathologic effects of artemisinin in broiler chickens following a single oral dose: an LD50 study. Int J Poultry Sci 8: 808-812.
- Gopalakrishnan A, Panicker VP (2014) An update on artemisinin- A multifaceted drug. Int J Pharm Tech Res 6: 1354-1361.
- Abdolmaleki Z, Arab HA, Amanpour S, Mansouri K, Tirgari F (2015) Assessment of anticancer properties of Artemisia sieberi and its active substance: an in vitro study. Basic Clin Cancer Res 7: 16-23.
- Abdolmaleki Z, Arab HA, Amanpour S, Muhammadnejad S (2016) Anti-angiogenic effects of ethanolic extract of Artemisia sieberi compared to its active substance, artemisinin. Rev Bras Farmacogn 26: 326-333.
- Mahboubi M, Farzin N (2009) Antimicrobial activity of Artemisia sieberi essential oil from central Iran. Iran J Microbiol 1: 43-48.
- Allen PC, Lydon J, Danforth HD (1997) Effects of components of Artemisia annua on coccidia infections in chickens. Poultry Sci 76: 1156-1163.
- Arab HA, Rahbari S, Rassouli A, Moslemi MH, Khosravirad F (2006) Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop Anim Health Prod 38: 497-503.
- Pop L, Gyorke A, Tabaran AF, Dumitrache MO, Kalmar Z, et al. (2015) Effects of artemisinin in broiler chickens challenged with Eimeria acervulina, E. maxima and E. tenella in battery trials. Vet Parasitol 214: 264-271.
- Ke OY, Krug EC, Marr JJ, Berens RL (1990) Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob Agents Chemother 34: 1961-1965.
- Kim JT, Park JY, Seo HS, Oh HG, Noh JW, et al. (2002) In vitro antiprotozoal effects of artemisinin on Neospora caninum. Vet Parasitol 103: 53-63.
- Medhi B, Patyar S, Rao RS, Byrav DS, Prakash A (2009) Pharmacokinetic and toxicological profile of artemisinin compounds: an update. Pharmacology 84:323-332.
- Hien TT, Hanpithakpong W, Truong NT, Dung NT, Toi P, et al. (2011) Orally formulated artemisinin in healthy fasting Vietnamese male subjects: a randomized, four-sequence, open-label, pharmacokinetic crossover study. Clin Ther 33: 644-654.
- Liersch R, Soicke H, Stehr C, Tullner HU (1986) Formation of artemisinin in Artemisia annua during one vegetation period,1. Planta Med 52: 387-390.
- Ashton M, Gordi T, Trinh NH, Nguyen VH, Nguyen DS, et al. (1998) Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm Drug Dispos 19: 245-250.
- Duc DD, de Vries PJ, Nguyen XK, Le Nguyen B, Kager PA, et al. (1994) The pharmacokinetics of a single dose of artemisinin in healthy Vietnamese subjects. Am J Trop Med Hyg 51: 785-790.
- Alin MH, Ashton M, Kihamia CM, Mtey GJ, Bjorkman A (1996) Clinical efficacy and pharmacokinetics of artemisinin monotherapy and in combination with mefloquine in patients with falciparum malaria. Br J Clin Pharmacol 41: 587-592.
- Ferreira JF, Gonzalez JM (2008) Chemical and biological stability of artemisinin in bovine rumen fluid and its kinetics in goats (Capra hircus). Rev Bras Parasitol Vet 17 Suppl 1: 103-109.
- Li C (2011) Study on artemisinin derivatives pharmacokinetics in dogs and metabolism in vitro. MSc Dissertation, Shanxi Medical School, China.
- Gordi T (2001) Clinical pharmacokinetics of the antimalarial artemisinin based on saliva sampling. Dissertation for the degree of PhD, Biopharmceutics and Pharmacokinetics, Uppsala University, Sweden.