Journal of Veterinary Science & Medicine
Download PDF
Research Article
Assessment of Bovine Tuberculosis like Lesions and Its Risk Factors in Cattle Slaughtered at Hawassa University and Municipal Abattoirs, Southern Ethiopia
Yibrah Tekle1*, Gezahegn Mamo2, Gobena Ameni3 and Mulugeta Ftiwi1
- 1College of Agriculture & Natural Resources, Department ofAnimal Science, Raya University, Maichew, Ethiopia
- 2College of Veterinary Medicine and Agriculture, Addis Ababa University, Maichew, Ethiopia
- 3Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Maichew, Ethiopia
*Address for Correspondence: Yibrah Tekle, College of Agriculture and Natural Resources, Raya University, Maichew, Ethiopia, E-mail: goytomtekle@gmail.com
Citation: Yibrah T, Gezehagne M, Gobena A, Mulugeta F. Assessment of Bovine Tuberculosis like Lesions and Its Risk Factors in Cattle Slaughtered at Hawassa University and Municipal Abattoirs, Southern Ethiopia. J Veter Sci Med. 2017;5(2): 9.
Copyright © 2017 Yibrah Tekle, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Veterinary Science & Medicine | ISSN: 2325-4645 | Volume: 5, Issue: 2
Submission: March 07, 2017 | Accepted: August 31, 2017 | Published: September 07, 2017
Abstract
Background: a cross sectional study was conducted on 753 Cattle, selected using systematic random sampling technique, in Hawassa abattoirs, Southern Ethiopia from December 2015 to May 2016 to investigate the occurrence of Bovine Tuberculosis in animals slaughtered at Hawassa University and Municipal Abattoirs, and to identifying the potential risk factors, Southern Ethiopia. The methods used were ante mortem, to identifying the risk factors, and postmortem examinations, to determining the bovine tuberculosis like lesions.
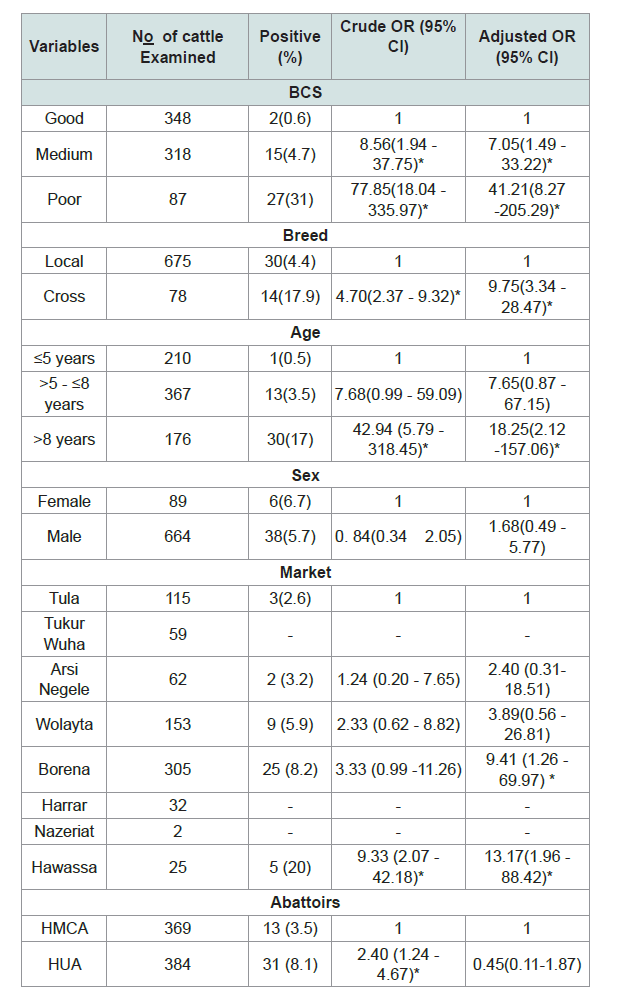
Result: the overall prevalence of the bovine tuberculosis was 5.8% (95% CI: 4.16-7.52) on the basis of detailed postmortem examination. Multivariable logistic regression analysis identified age, body condition; breed and market were statistically significant. The older cattle above 8 years were eighteen times (OR = 18.25) more likely to have tuberculosis than the younger cattle. The cross breeds were nine times (OR = 9.75) more sensitive to M. bovis as compared to local breeds as well as the poor body conditioned cattle forty-one times (OR = 41.21) more likely to have tuberculosis than the cattle have good body condition. Relatively, the occurrence of BTB was higher in cattle brought from Hawassa (OR = 13.17) and Borena (OR = 9.41) markets than Tula market. The lesions were found most frequently distributed in thoracic cavity lymph nodes (75%), which indicated that respiratory route was the main mode of infection in the study area.
Conclusion: Using the gross pathological lesion this study shows that low overall prevalence of bovine tuberculosis in sampled animals.
Keywords
Ante and post mortem examinations; Bovine tuberculosis like lesions; Hawassa abattoirs; Risk factors
Introduction
Tuberculosis (TB) is a chronic pulmonary disease which threats to animal and human causing high morbidity, economic losses and mortality [1-3]. Since TB is a major health threats particularly in developing countries, it has been declared as global emergency by the World Health Organization in 1995[4]. TB causes death in each year among millions of people as a result it ranked as globally a second leading disease from the infectious disease next to human immunodeficiency virus (HIV)[5]. TB is a leading killer among adults in the most economically productive age groups and people living with HIV, and even those cured from TB can be left with lifetime sequelae that substantiallyreduce their quality of life [6,7].
Bovine tuberculosis (BTB) is among the principal zoonotic diseases caused by M. bovis which affects many vertebrate animals and humans and characterized by progressive development of granulomas/ tubercles in tissues and organs [8-15]. Bovine tuberculosis has been widely distributed throughout the world and has been recognized from 176 countries as one of the important bovine diseases causing great economic loss in animal production [16].
Bovine tuberculosis affects broad range of mammalian alian hosts including humans, cattle, deer, pigs, domestic cats, wild carnivores and omnivores [17]; it rarely affects equids or sheep [18,19]. Moreover, human TB of animal origin caused by M. bovis is becoming increasingly evident in developing countries [20,21]. The productivity efficiency of the infected animal reduced from 10-25%; the direct losses due to the infection decreases in milk production 10-18% and meat production 15%. BTB has both the effect in animal production and public health importance [22,23].
In developing countries like Ethiopia, the low standard living areas and socio-economic situation for both animals and humans are more contributing in TB transmission between human to human and human to cattle or vice versa [24,25]. Organisms are excreted in the exhaled air, in sputum, feces (from both intestinal lesions and swallowed sputum from pulmonary lesions), milk, urine, vaginal and uterine discharges, and discharges from open peripheral lymph nodes of infected animals [19,26]. Human infection by M. bovis is thought to be mainly through drinking of contaminated or unpasteurized raw milk. The potential for transmission of M. bovis and other mycobacteria between cattle and humans are the presence of close contact of animal and humans or the rural societies living together with their animals in the same microenvironment and house, raw milk and meat consumption habit, the prevalence of HIV increasing and HIV patient’s susceptibility to TB [27]. In the areas where the bovine tuberculosis is common and the milk pasteurization is rare, M. bovis cases in human estimated 10-15% [28,29]. Thus, in cattle the main route of infection transmission: aerosol, close contact betweenanimals and ingestion of contaminated products [30-33].
Bovine tuberculosis, is an endemic disease of cattle in Ethiopia with prevalence of 1.1%- 24.7% in abattoir and 3.5-50% in crossbreed farms [27,34-36], like human TB has not received more focus on research and its control strategies plus the test and slaughter control strategies not applied, due to its economic constraints, but its applicable effective method in developed countries [37]. The epidemiology BTB is not well identified in livestock, inadequate comprehensive abattoir surveillance and lack of diagnostic facilities the BTB has limited information and the majority researches were focused at the central part of Ethiopia [38,39]. Hence, the epidemiology of BTB has to be assessed widely in all regions in order to embark the national BTB control program in the future. Therefore, this study was designed to assess the prevalence of bovine tuberculosis based on postmortem examination of slaughtered cattle at Hawassa University and municipal abattoirs and to evaluate the association between the potential risk factors and bovine tuberculosis.
Materials and Methods
Study area
The study was conducted from December 2015 to May 2016 in Hawassa University and municipal abattoir in Southern Nations Nationalities and Peoples Regional state (SNNPRs), Southern Ethiopia. This region has an estimated total area of about 112,323.19 km2. Hawassa city is located in Southern part of Ethiopia, in Sidama Zone, on the shores of Lake Hawassa in the Great Rift Valley and located 270 km South of Addis Ababa. The city serves as the capital of the SNNPRs, and its total area is 157.21 square kilometers. It bounded by Lake Hawassa on the West and North-west, Chelelaka swampy area on the East and South-East, Tikur Wuha River on the North and Alamura Mountain on the South. It lies on the Trans-African Highway for Cairo-Cape Town, and has a geographic coordinate of 7 °3′ N latitude and 38 °28′ E longitude and an elevation of 1708 m.a.s.l. [40].The livestock resource of the city is 61,123 cattle, 14,764 sheep, 17,735 goats, 5,544 equines and 56,961 poultry and the total population is estimated about 304,479 [41].
Hawassa city has two abattoirs: one municipality abattoir and second Hawassa University abattoir, in main campus. The Hawassa municipal abattoir supplies the inspected meat to about 304,479 inhabitants and based on the information obtained from personnel working in Hawassa University Registrar Office, the University’s abattoir supplies for about 25,000 students (personal communication) [41].
Even though the abattoirs were fenced, the places used to ispose condemned carcasses were not secured since they were easily accessed by hyenas, dogs and other animals. The overall hygiene and the internal facilities including the drainage were not good in the Municipal abattoir whereas the University’s abattoir was good. Even though the Municipal abattoir has recently built abattoir inside the compound, currently the slaughtering is performing in the old house. The minimum and maximum numbers of cattle slaughtered per day during the study period in Hawassa city Municipal abattoir were about 75 and 175 heads of cattle, respectively and about 8 and 12 heads of cattle were for Hawassa University abattoir, respectively. Few numbers of female cattle were also slaughtered in both abattoirs. The municipal abattoir was operated by one veterinarian and three assistant meat inspectors but the University abattoir was operated only by one assistant meat inspector (Personal communication to the responsible bodies).
Study animal
A total of 753 apparently healthy adult cattle slaughtered in the abattoirs (Hawassa city municipal abattoir, n = 369 and Hawassa University abattoir, n = 384) regardless of sex, breed and origin were considered in the study. And the cattle used for this study were mainly originated from different markets (source of cattle); for the municipal abattoir were from Tula, Arsi Negele, Tukr Wuha, Wolayita, Harrar, Borena, Hawassa and Nazeriat (Adama) Markets and for University abattoir the origin were from Borena and Wolayita markets. Basic animal information on each study animals (such as sex, age, breed, body condition score, market) were collected and recorded during ante mortem examination. All the study animals in this study were cross and local breed.
Study design and sampling method
A cross sectional study with systematic random sampling was carried out to examine the carcass and sample suspected TB lesions from cattle slaughtered at Hawassa city municipal and Hawassa University abattoirs.
In these abattoirs, individual heads of cattle to be slaughtered were given identification number for proper recording of ante mortem and post mortem examination results. Selection of cattle population to be involved in the study was based on systematic random sampling, from among those cattle slaughtered each day, which was arranged in a systematic manner with each individual animal constituting a sampling unit. Systematic random selection of animals was made based on the arrangement that the population (cattle slaughtered each day) was first arranged in groups based on sampling interval (batch). This sampling interval was obtained by dividing the total animals slaughtered within that day by the estimated daily sample size. Each individual animal was then selected randomly from the first sampling interval (batch), then, every nth animal from the subsequent batch was included in the sample.
In Hawassa city municipal abattoir on average 20 carcasses were decided to be inspected each day (range from 15 to 25) from among those slaughtered cattle whereas in Hawassa University abattoir the whole cattle slaughtered in each day were considered for postmortem examination because the number of slaughter cattle was often fewer (range from 6 to 12) compared the Hawassa city municipal abattoir. The work was carried out in all the weekdays except Wednesday and Friday when animals were not slaughtered due to fasting occasions according to the belief of Ethiopian Orthodox Christianity.
Sample size determination
All animals coming to the abattoirs from different markets during the study period were considered for sampling. The sample size calculation was done by using the Thrusfield formula [42]:
Where n = required sample size
Pexp = expected prevalence, 8.8% was for the Hawassa city municipal abattoir d = desired absolute precision, 5%
The expected prevalence, Pexp, of Hawassa city municipal abattoir was (8.8%) used from the previous works [43]. The calculated sample size for the municipal abattoir was 123 heads of cattle, but to increase the precision of the study this sample size multiplied three times, then n = 369 heads of cattle. Whereas the calculated sample size of the Hawassa University abattoir, Pexp considered as 50% because there was no previous study on bovine tuberculosis in this abattoir, was 384 heads of cattle. Thereafter, the total animals supervised in both abattoirs were 753 heads of cattle.
Ante mortem examination
Clinical examination was conducted on the study animals before they slaughtered; this included examining of superficial lymph nodes, visible mucus membrane, and other basic parameters [44]. Age determination based on dentition (teeth) as previously described [45,46] and the body condition of each of the study animal was scored using the guidelines established by Nicholson and Butterworth and Maurya et al. [47,48]. Accordingly, on the basis of observation of anatomical parts such as vertebral column, ribs, and spines, the study animals were classified as poor (score, 1 to 3), medium (4 to 6), or good (greater than 6). The breed, sex and source of the animals (markets) were recorded during ante mortem examination.
Post mortem examination
Post mortem examination was carried our according to the OIE and Meat Inspection and Quarantine Division of the Ministry of Agriculture method [49,50]. All lymph nodes, livers, kidney and lungs were visualized, palpated, and incised into a size of 2 mm to facilitate the detection of tuberculous lesion from each animal. These include the mandibular, medial retropharyngeal, cranial and caudal mediastinal, left and right bronchial, hepatic, mesenteric lymph nodes as well as the seven lobes of the two lungs, including the left apical, left cardiac, left diaphragmatic, right apical, right cardiac, right diaphragmatic and right accessory lobes were investigated. The animal was classified as suspected tuberculos lesion when tuberculous lesion was found, and if not as non-lesioned.
The severity of gross lesions in individual lymph node was scored as follows; 0 = no gross lesion, 1 = small lesion at one focus, 2 = small lesions at more than one focus and 3 = extensive necrosis as developed by Vordermeier et al. and Ameni et al. [51,52]. And for organs, the pathological scoring was scored separately as follows: 0 = no visible lesions; 1 = no gross lesions but lesions apparent on slicing of the lobe; 2 = fewer than five gross lesions; 3 = more than five gross lesions; 4 = gross coalescing lesions. The scores for the individual lobes were added up to calculate the lung score. The cut surfaces were examined under bright light for the presence of abscess, cheesy mass, and tubercles [53].
Statistical analysis
Prevalence of BTB was calculated as the proportion of suspected lesion positive animals from the total number of animals sampled [42]. Data related with age, sex, breed, markets source of animals and body conditions scoring of each animal were recorded on a data sheet during ante mortem examination. The recorded data was entered, stored, classified, coded and filtered using Microsoft Excel computer program and was transferred and analyzed by STATA version 11 (STATA Corp. College station, TX). Presence or absence of TB like lesions and affected tissues (suspected lesions) were recorded during postmortem examination. The variations between different factors were analyzed using logistic regression and chi-square (χ2) was used for association of different risk factors. P-value less than 5% was considered statistically significant. In cases of estimating the effect of different risk factors in terms of odds ratio (OR), to assess the strength of association of different factors with the prevalence of BTB, with corresponding 95% confidence interval, statistical significance was assumed if the confidence interval did not include one among its values
Result
Prevalence and associated risk factors analysis
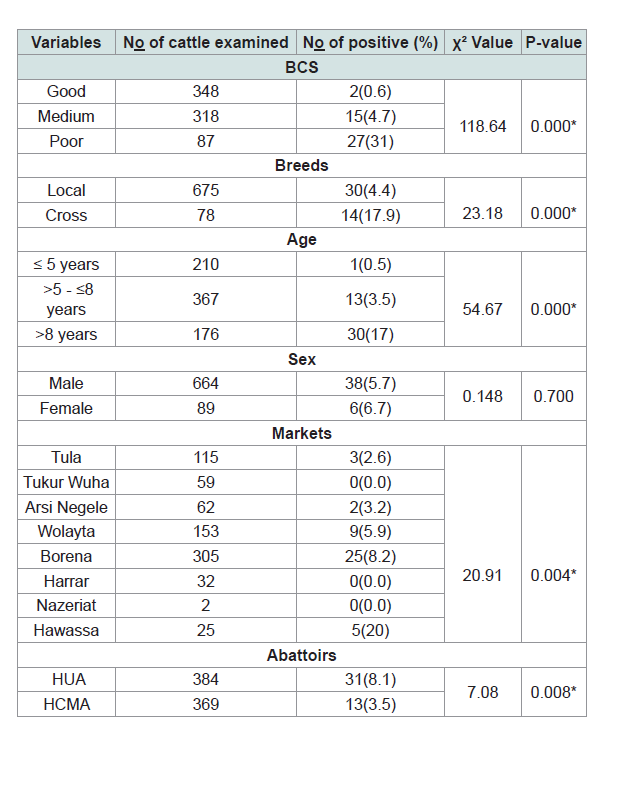
Upon detailed post mortem examination of 753 cattle, an overall prevalence of BTB was 5.84% (95% CI: 4.58 - 8.42). Out of the total 753 slaughtered cattle in both abattoirs, 675 (89.6%) were local breed and 78 (10.4%) cross breed whereas the prevalence of tuberculous lesion was higher in cross breed, 17.9% than local breeds, 4.4% and this difference in prevalence of BTB was found to be statistically significant (χ2 = 23.18, P = 0.000). Associations of the risk factors, the difference in prevalence of BTB in relation to body condition score, age, and markets were statistically significant (χ2 = 118.64, p = 0.000; χ2 = 54.67, p = 0.000 and χ2 = 20.9, p = 0.004) respectively (Table 1).
Multivariable logistic regression analysis identified age, body condition; breed and market were statistically significant (Table 2).
Table 1:Association of different risk factors with gross pathological lesion of bovine tuberculosis in Hawassa abattoirs, Southern of Ethiopia.
Table 2:Univarite and multivariable logistic regression analysis of bovine tuberculosis lesion with various host related risk factors.
The cross-breed cattle (OR = 9.75, 95% CI: 3.34 - 28.47) slaughtered in the abattoirs nine times more sensitive to M. bovis than the local breeds and also the cattle brought from Hawassa and Borena markets had more likely bovine tuberculosis lesions thirteen times (OR = 13.17, 95% CI: 1.96 - 88.42) and nine times (OR = 9.41, 95% CI: 1.26 - 69.97) respectively than Tula market (Figure 1). Older cattle above 8 years had eighteen times the odds of being bovine tuberculosis lesion compared with the younger age group (5 or less than 5 years old) (OR = 18.25; 95% CI: 2.12 - 157.06). In relation to sex and abattoirs, there were no statistical significance differences of bovine tuberculosis lesion positive between groups.
Distribution of gross pathological lesions
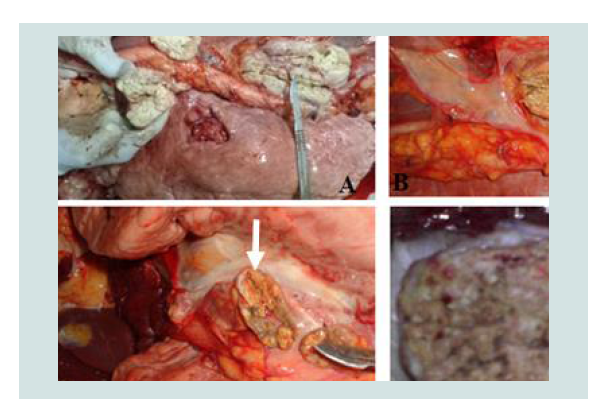
Gross pathological lesions were observed in lymph nodes and organs of the slaughtered cattle; the majority of the lesions were considered typical of tuberculous lesions which characterized by central round, oval, or irregular, often coalescing areas of caseous necrosis and mineralization (calcification) (Figure 2). Large encapsulated nodules containing thick yellowish cheesy material were mostly observed in the thoracic lymph nodes. Whenever gross lesions of suggestive pathological lesions of TB noticed in any tissue; the tissue was classified as positive for TB.
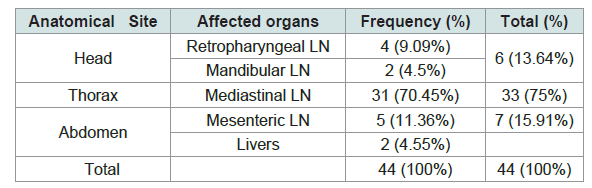
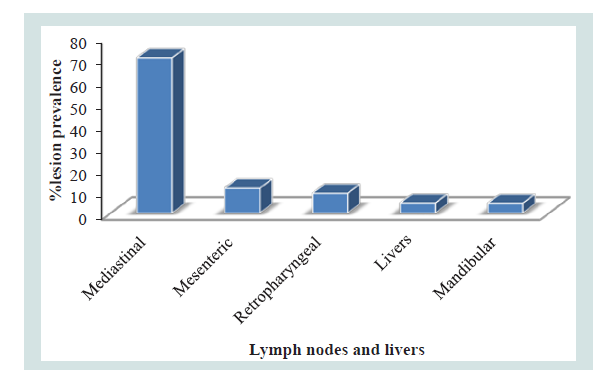
According to the anatomical site, 75% (33/44) of the gross lesions were sampled from thoracic cavity followed by abdomen cavity and head region 13.64% (6/44) and 15.91% (5/44) respectively. 70.45% (31/44) of the gross lesions were collected from Mediastinal lymph nodes whereas only 11.36% (5/44) where obtained from mesenteric lymph nodes (Table 3 and Figure 3).
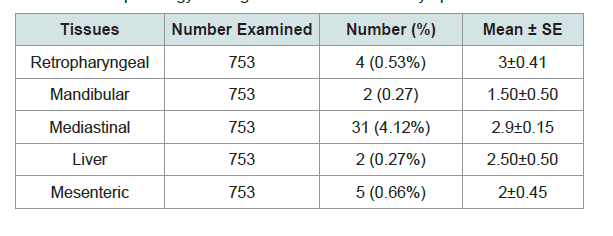
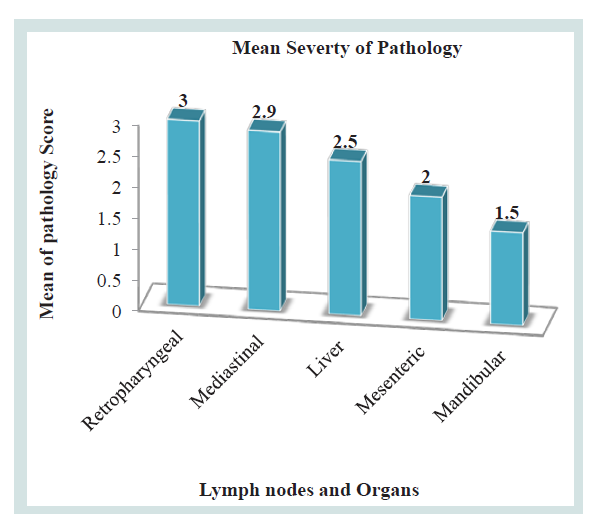
The pathological scoring analysis of the lymph nodes and organs described by the mean severity of lesion; retropharyngeal lymph nodes (3±0.408) was higher followed by mediastinal lymph node (2.9±0.149), Liver (2.5±0.50), mesenteric lymph nodes (2±0.447), mandibular (1.50±0.50) Table 4 and Figure 4).
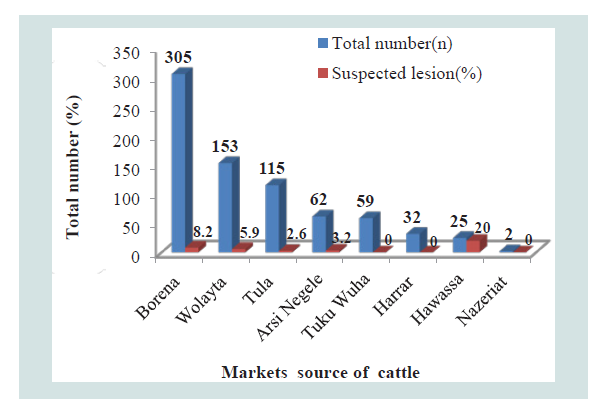
Figure 1:The markets total number of cattle slaughtered and their proportion of suspected lesions in Hawassa abattoirs, Southern Ethiopia.
Figure 2:The typical TB lesions of cattle slaughtered in Hawassa city unicipal abattoir (B & A) and Hawassa University abattoir (D & C); B, C & D = calcified and granulomatous lesion in mediastinum lymph nodes, A = Caseous and granulomatous necrosis from mediastinum lymph nodes (lesions indicated by white arrow).
Discussion
Bovine tuberculosis is a chronic infectious disease of animals characterized by the formation of granulomas in tissues and its detection is carried out most commonly on the basis of tuberculin skin testing, abattoir meat inspection and rarely on bacteriological techniques. Bovine tuberculosis has serious economic significance to the livestock sector and public health hazard to human. Tuberculosis caused by M. bovis is clinically indistinguishable from tuberculosis caused by M. tuberculosis and the proportion of human tuberculosis caused by M. bovis is estimated to 10-15% [28,29].
Bovine tuberculosis, caused by M. bovis, is known to be endemic of most developing countries including Ethiopia although the magnitude varies between livestock production system and breed of animals still nationwide epidemiological surveillance and control activities are often inadequate or unavailable in most developing countries [32,38,52].
In the present study, the overall prevalence of BTB was 5.84% (95% CI: 4.58 - 8.42) which is comparably in agreement with the findings of various researchers who reported prevalence of BTB 4.2% in Yabello municipal, 4.5% in Hosaana abattoir , 5.16% in Adama Municipality abattoir , 5.1% in Nekemte Municipality abattoir, 5% in Kombolcha ELFORA abattoir , 6.4% in Mekelle town municipal abattoir and 6.79% in Adama municipal abattoir as well as it was the same (5.8%) with research report of Romha et al. in western Tigray Zone [43,54-60]. However, the total prevalence of this study was lower than previous studies carried out by other authors; 11.50% by Abdurohaman in Butajira, 9% Nemomsa et al. in Butajira abattoir, 8.8% by Biffa et al. in Hawassa municipal abattoir, 7.96% by Regassa in Wolayta, Southern Ethiopia and 24.7% in Adama municipal abattoir [35,61-63]. On the other hand, the finding of this study was higher than the results of Regassa et al. in Hawassa municipal abattoir (1.1%), Gebremedhin et al. in Dilla Municipal Abattoir (2.6%), Asseged et al. in Addis Ababa (1.48%) and Shitaye et al. in Addis Ababa (3.46%) [36,64-66]. This lower prevalence recorded in the present study could be due to the fact that animals slaughtered in the abattoirs were mainly local breeds (Zebu) (675 out of 753) which are relatively resistant to BTB [32]. This the variations in prevalence could be due to the possible difference in the epidemiology of the disease in the animal populations, markets sources of animal (from which they brought to abattoirs either from high BTB prevalent or their local BTB burden), body condition score of the animals and types of production system; The intensive livestock management system could contribute the development of mycobacterial infections than the extensive livestock management system [21,26,32].
The prevalence of BTB among the markets (source of cattle) and between the two abattoirs was statistically significant (P = 0.004, p = 0.008 respectively). Based on the post mortem inspection, the prevalence of TB lesions showed marked variation between the two abattoirs; the cases recorded in cattle slaughtered in Hawassa university abattoir was higher, 8.1% (31/384) than Hawassa municipal abattoir, 3.5% (13/369). Because most of the animal slaughtered at Hawassa University abattoir were from Boren area which might show the high prevalence in the source [67]. The abattoirs have no effects in the development of the TB infection within that short period of time since TB is a chronic disease that needs long period of time. For this reason, the statistically significance of the abattoirs indicated that to the prevalence variation of the market source of cattle.
The prevalence of BTB of cattle brought from various markets which were source of cattle to both abattoirs was different; higher prevalence was observed in the cattle come from Hawassa market 20% (5/25); followed by the markets of Borena 8.2% (25/305), Wolayta 5.9% (9/153), Arsi Negele 3.2% (2/62) and Tula 2.6% (2/115) but there was no BTB in the cattle brought from the Tukur Wuha, Harrar and Nazeriat markets. The source of cattle for Hawassa University abattoir were from Borena (275/305) and Wolayta (109/153) markets and also the source of cattle for Hawassa city municipal abattoir were from Borena (30/305), Wolayta (44/153), Tula (115/115), Tukur Wuha (59/59), Harrar (32/32) and Nazeriat (2/2) markets. The cattle purchased from Hawassa market was (OR=13.17) thirteen times more likely to have the BTB than those cattle bought from Tula market. The infection rate in cattle has been found to differ greatly from place to place and the difference most probably linked to the type of production system (extensive), which is unlikely to favor the spread of the disease in contrast to the intensive dairy farms Grazing on the open field reduces the level of confinement and in turn minimizes the rate of infection in the herd [52,66,68].
The prevalence of bovine tuberculosis in this study showed a statistically significant difference among the age groups (P = 0.000). This result is in consistent with the reports of Gebremedhin et al. in Dilla Municipal Abattoir, Nemomsa et al. and Hussein in Butajira abattoir [64,62,69]. The occurrences of TB lesions in cattle were 68.2% (30/44) in the older cattle (greater than 8 years) than younger cattle, 2.3% (1/44) and adult cattle, 29.5% (13/44), age groups; this indicted as the age of the cattle increased the prevalence also increased. The older cattle (OR = 18.25) were eighteen times more likely to have the gross pathological lesions than the younger cattle. The results of the current research also agreed with findings of Barwinnek and Taylor , Ameni et al., Regassa et al. and Biffa et al. that explained as the age of the cattle increases the probability of acquiring TB infection also increases [70,32,36,71]. The reason can be by declining of protective capability in aging animals, have weaker immune system [72]; this is due to the fact that stresses, malnutrition and immunosuppression increase with age [73].
The difference in the prevalence of BTB among animals having different body condition scores was statistically significant (P = 0.000) and the prevalence was highest in cattle with poor body condition (31%) as compared to cattle with medium body condition (4.7%) and good body condition cattle (4.5%) which is in agreement with study resulted by Nemomsa [62]. The poor body conditioned cattle were (OR = 41.21) forty one times more likely to have the BTB compare to the good body conditioned cattle. This could due to related to the weak protective immune response in poor body conditioned cattle compared to good ones that may result extensive lesions and wasting of the body condition as well as its chronicity nature of the disease. The present result is in consistent with previous reports which indicated that animals with good body condition haverelatively good immunological response to the infectious agent thananimals with medium and poor body condition score [22,26,74].
Variation among breeds in susceptibility to tuberculosis has been documented in Ethiopia and elsewhere and the result of this study agreed; was statistical significant between cattle breed and tuberculous lesion (P = 0.000) [52,75-77]. The prevalence of the BTB was being highest in cattle cross breed 10.36% (14/78) compared to cattle local breeds, 4.4% (30/675). The cross breeds (OR = 9.75) were nine times more sensitive to M. bovis (owned BTB lesions) as compared to local breeds. The finding of lower prevalence in the local/Zebu breed is in line with other previous studies which showed different breeds could result in difference in susceptibility to BTB infections. The difference susceptibility of BTB infection between breeds is likely to be related to differences in management: those genetically improved cattle are more prone to BTB infection than local breeds since they may suffer more severely from deficient housing and malnutrition [22,,58,72,78-81].
The difference in the prevalence of BTB lesion between the two sexes was statistically insignificant (P = 0.700). BTB suspected slaughtered female and male cattle were 6.7% (6/89) and 5.7%(38/664) respectively. This insignificant result is in consistent with previous studies [33,54,65,76]. The possible reason might be due to in proportionality in the number of female and male animals compared in specific variable; less number of female animals was come to the study abattoir to be slaughtered.
The best evidence of the transmission route of M. bovis to cattle is the pattern of lesions observed in slaughtered animals [19]. In the present study, gross tuberculous lesions were found most frequently in lymph nodes of the thoracic cavity, 75% (33/44); followed by the lymph nodes of the head region, 13.64% (6/44) and the lymph nodes of the abdominal cavity 11.36% (5/44). The occurrence of tuberculous lesions in thoracic cavity was lower than the results of previous studies which reported greater than 84% TB lesions occurrence in the respiratory system [54,74,82-84]; whereas it was higher than the report of Dechassa (67.7%), Firdessa (70%), Miliano-suazo et al. (49.2%) and Regassa et al. (50%) [59,85,86,36]. As a result, this study indicated the main route of transmission and infection being respiratory route and this finding agreed with the previous researchers who reported the same route of transmission and infection, respiratory route [19,36,54,55,82,87,88].
Conclusion and Recommendations
The output of this study has indicated that an overall BTB prevalence of 5.8% of which 20%, 8.2%, 5.9%, 3.2% and 2.6% were recorded in these sampled animals come from Hawassa, Borena, Wolayta, Arsi Negele and Tula markets respectively. Even though the overall prevalence was low, high prevalence was found in animals which are older, poor body condition and cross breeds cattle. Similarly, relatively high occurrence of BTB was in cattle brought from Hawassa and Borena markets than Tula market. This could be indicating the presence of BTB infection in certain geographical areas. This research also shown that the respiratory route was the major means of BTB transmission among the cattle population and the higher mean severity of pathology among associated lymph nodes and organs was recorded in retropharyngeal and mediastinal lymph nodes. In conclusion, in this study the gross pathological lesion findings could indicate the occurrence of BTB in those apparently healthy sampled cattle, a threat to livestock production and also for public health. Basis of findings the present study the following points are recommended:
Further investigation should be done by including wide geographical areas and large sample size to elucidate the epidemiological information on circulating strains, potential risk factors, ways of transmission and molecular diversity of the M. bovis strains as well as their zoonotic role in human.
A proper postmortem meat inspection should be practiced efficiently in the abattoirs before taking beef to the retail markets to reduce the public health risk.
References
- Abdurohaman M (2009) Cross sectional study of bovine tuberculosis in Butajira municipal abattoir, South West Ethiopia. J Global Vet 6: 172-179.
- Amanfu W (2006) The situation of tuberculosis and tuberculosis control in animals of economic interest. Tuberculosis (Edinb) 86: 330-335.
- Chimdi GA, François R (1998) Study on the epidemiology of bovine tuberculosis in dairy farms (Debre Zeit and Ziway, Ethiopia). In: Proceeding of the 12th Conference. EVA, Addis-Ababa, Ethiopia. pp. 13-19.
- Ameni G, Wudie A (2003) Preliminary study on bovine tuberculosis in Nazareth municipality abattoir of central Ethiopia. B Anim Health Pro Afr 51: 25-132.
- Ameni G, Aseffa A, Engers H, Young D, Hewinson G, et al. (2006) Cattle husbandry in Ethiopia is a predominant factor affecting the pathology of bovine tuberculosis and gamma interferon responses to mycobacterial antigens. Clin Vaccine Immunol 13: 1030-1036.
- Ameni G, Aseffa A, Engers H, Young D, Gordon S, et al. (2007) High prevalence and increased severity of pathology of bovine tuberculosis in Holsteins compared to zebu breeds under field cattle husbandry in central Ethiopia. Clin Vaccine Immunol 14: 1356-1361.
- Ameni G, Desta F, Firdessa R (2010) Molecular typing of Mycobacterium bovis isolated from tuberculosis lesions of cattle in north eastern Ethiopia. Vet Rec 167: 138-141.
- Ashford DA, Whitney E, Raghunathan P, Cosivi O (2001) Epidemiology of selected mycobacteria that infect humans and other animals. Rev Sci Tech 20: 325-337.
- Asseged B, Lubke-Beeke A, Lemma ETadele K, Britton S (2000) Bovine tuberculosis: a cross-sectional and epidemiological study in and around Addis ababa. Bull Anim Health Prod Afr 48: 71-80.
- Asseged B, Woldesenbet Z, Yimer E, Lemma E (2004) Evaluation of abattoir inspection for the diagnosis of Mycobacterium bovis infection in cattle at Addis Ababa abattoir. Trop Anim Health Prod 36: 537-546.
- 11.Awah-Ndukum J, Kudi AC, Bradley G, Ane-Anyangwe I, Titanji VP, et al. (2012) Prevalence of bovine tuberculosis in cattle in the highlands of Cameroon based on the detection of lesions in slaughtered cattle and tuberculin skin tests of live cattle .Vet Med 57: 59-76.
- Ayele WY, Neill SD, Zinsstag J, Weiss MG, Pavlik I (2004) Bovine tuberculosis: an old disease but a new threat to Africa. Int J Tuberc Lung Dis 8: 924-937.
- Barwinnek F, Taylor NM (1996) Assessment of the socio-economic importance of bovine tuberculosis in Turkey and possible strategies for control or eradication. Turkish-German Animal Health Information Project. General directorate of protection and control, Ankara. Eschborn: Deutsche Gesellschaft fur Technische Zusammenarbeit. pp. 3-45.
- Berg S, Firdessa R, Habtamu M, Gadisa E, Mengistu A, et al. (2009) The burden of mycobacterial disease in Ethiopian cattle: implications for public health. PLoS One 4: e5068.
- Demelash B, Inangolet F, Oloya J, Asseged B, Badaso M, et al. (2009) Prevalence of bovine tuberculosis in Ethiopian slaughter cattle based on post-mortem examination. Trop Anim Health Prod 41: 755-765.
- Biffa D, Bogale A, Skjerve E (2010) Diagnostic efficiency of abattoir meat inspection service in Ethiopia to detect carcasses infected with Mycobacterium bovis: implications for public health. BMC Public Health 10: 462.
- Biffa D, Skjerve E, Oloya J, Bogale A, Abebe F, et al. (2010) Molecular characterization of Mycobacterium bovis isolates from Ethiopian cattle. BMC Vet Res 6: 28.
- Biffa D, Inangolet F, Bogale A, Oloya J, Djønne B, et al. (2011) Risk factors associated with prevalence of tuberculosis-like lesions and associated mycobacteria in cattle slaughtered at public and export abattoirs in Ethiopia. Trop Anim Health Prod 43: 529-538.
- Bonsu OA, Laing E, Akanmori BD (2000) Prevalence of tuberculosis in cattle in the Dangme-West district of Ghana, public health implications. Acta Trop 76: 9-14.
- Buncic S (2006) Integrated food safety and veterinary public health. CABI, London, UK, 61.
- Chauhan RS, Agarwal DK (2006) Text book of veterinary clinical and laboratory diagnosis. (2nd Edn), New Delhi, India.
- Chukwu ID, Chukwu CO, Kandakai-Olukemi YT, Owolodun O, Nwosuh C, et al. (2013) Detection of Mycobacterium tuberculosis complex in lung specimen of slaughtered cattle and goats by a DNA based multiplex polymerase chain reaction and ziehl-neelsen methods in Jos, Nigeria. Br Microbiol Res J 3.
- Cleaveland S, Shaw DJ, Mfinanga SG, Shirima G, Kazwala RR, et al. (2007) Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis (Edinb) 87: 30 - 43.
- Collins CH, Grange JM (1994) The bovine tubercle bacillus. J Appl Bacteriol 55: 13-29.
- Collins JD (1996) Factors relevant to M. bovis eradication. Irsh Vet J 49: 241-243.
- Corner L, Melville L, McCubbin K, Small KJ, McCormick BS, et al. (1990) Efficacy of inspection procedures for detection of tuberculous lesions in cattle. Aust Vet J 67: 338-392.
- Corner LA (1994) Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet Microbiol 40: 53-63.
- Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, et al. (1998) Zoonotic tuberculosis due to M. bovis in developing countries. Emerg Inf Dis 4: 59-70.
- ISHN (2007) Ethiopia - population and housing census of 2007. Addis Ababa, Ethiopia.
- Federal Democratic Republic of Ethiopia, Central Statistical Agency (2008) Agricultural sample survey: report on livestock and livestock characteristics (private peasant holdings) 2, Addis Ababa.
- Dechassa T (2014) Gross pathological lesions of bovine tuberculosis and efficiency of meat inspection procedure to detect-infected cattle in Adama municipal abattoir. J Vet Med Anim Health 6: 48-53.
- de Lisle GW, Bengis RG, Schmitt SM, O’Brien DJ (2002) Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Rev Sci Tech 21: 317-334.
- Delahay RJ, De Leeuw AN, Barlow AM, Clifton-hadley RS, Cheeseman CL (2002) The status of Mycobacterium bovis infection in UK wild mammals: a review. Vet J 164: 90-105.
- DeLahunta A, Habel RE (1986) Applied veterinary anatomy. Saunders Company, USA.
- Desta F (2008) Study on Mycobacterium bovis using conventional and molecular methods in cattle slaughtered in Kombolicha ELFORA meat processing plant. Addi Ababa University, Ethiopia.
- Ejeh EF, Markus IF, Ejeh AS, Musa JA, Lawan FA, et al. (2013) Seasonal prevalence of bovine tuberculosis lesion in cattle slaughtered in Yola abattoirs. Bangladsh J Vet Med 11: 113-120.
- Elias K, Hussein D, Asseged B, Wondwossen T, Gebeyehu M (2008) Status of bovine tuberculosis in Addis Ababa dairy farms. Rev Sci Tech 27: 915-923.
- Firdessa R (2006) Preliminary study on bovine tuberculosis in and around Sululta town, North shoa zone of Oromia, Faculty of Vetrinary Medicin, Addis Ababa University.
- Gebrezgabiher G, Romha G, Ameni G (2014) Prevalence study of bovine tuberculosis and genus typing of its causative agents in cattle slaughtered at Dilla Municipal Abattoir, Southern Ethiopia. Afr Basic Appl Sci 6: 103-109.
- Goodchild AV, Clifton-Hadley RS (2001) Cattle-to-cattle transmission of Mycobacterium bovis. Tuberculosis (Edinb) 81: 23-41.
- Grange JM, Yates MD, Kantor IN (1996) Guidelines for speciation within the Mycobacterium tuberculosis complex (2ndedn), World Health Organization.
- Jaleta TG (2008) Study on bovine tuberculosis in Nekemte municipality abattoir, Western Ethiopia, College of Agriculture and Veterinary Medicine, School of Veterinary Medicine, Jimma University, Jimma, Ethiopia.
- Hailemariam S (1975) A brief analysis of activities of meat inspection and quarantine division Department of Veterinary Service, Ministry of Agriculture, Addis Ababa, Ethiopia.
- Hlokwe TM, van Helden P, Michel A (2013) Evaluation of the discriminatory power of variable number of tandem repeat typing of Mycobacterium bovis isolates from Southern Africa. Tranbound Emerg Dis 60 Suppl 1: 111-120.
- Humblet MF, Boschiroli ML, Saegerman C (2009) Classification of worldwide bovine tuberculosis risk factors in cattle: a stratified approach. Vet Res 40: 1-24.
- Kazwala RR, Kambarage DM, Daborn CJ, Nyange J, Jiwa SF, et al. (2001) Risk factors associated with the occurrence of bovine tuberculosis in cattle in the Southern highlands of Tanzania. Vet Res Commun 25: 609-614.
- Kiros T (1998) Epidemiology and zoonotic importance of bovine tuberculosis in selected sites of Eastern Shoa Ethiopia, Faculties of Veterinary Medicine, Addis Ababa University and Freie Universitaet Berlin.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367: 1747-1757.
- Mamo G, Abebe F, Worku Y, Hussein N, Legesse M, Tet al. (2013) Bovine tuberculosis and its associated risk factors in pastoral and agro-pastoral cattle herds of Afar Region, Northeast Ethiopia. J Vet Med Anim Health 5: 171-179.
- Maurya VP, Sejian V, Naqvi S (2009) Body condition scoring system-a strategic tool for optimizing productivity in animal farm. Indian Farming 58: 28-33.
- Mbugi EV, Katale BZ, Kendall S, Good L, Kibiki GS, et al. (2012) Tuberculosis cross-species transmission in Tanzania: towards a one-health concept. Onderstepoort J Vet Res 79: 501.
- Menzies FD, Neill SD (2000) Cattle-to-cattle transmission of bovine tuberculosis. Vet J 160: 92-106.
- Miliano-suazo , Salmar MD, Ramirez C, Payeur JB, Rhyan SC, Santillan M (2000) Identification of TB in cattle slaughtered in Mexico. Am J Vet Res 61: 86-87.
- Miller TL, McNabb SJ, Hilsenrath P, Pasipanodya J, Weis SE (2009) Personal and societal health quality lost to tuberculosis. PLoS One 4: e5080.
- 55.Zeru F, Romha G, Ameni G (2013) Gross and molecular characterization of Mycobacterium tuberculosis complex in Mekelle town municipal Abattoir, Northern Ethiopia. Glob Vet 5: 541-546.
- Romha G, Ameni G, Berhe G, Mamo G (2013) Epidemiology of mycobacterial infections in cattle in two districts of Western Tigray Zone, Northern Ethiopia. AFJMBR 7: 4031-4038.
- Nemomsa B, Gebrezgabiher G, Birhanu T, Tadelle H, Tadesse G (2014) Epidemiology of bovine tuberculosis in Butajira, Southern Ethiopia: a cross-sectional abattoir-based study. Afr J Microbiol Res 33: 3112-3117.
- Regassa A (1999) Preliminary study on bovine tuberculosis in Wolaita-Sodo, South Ethiopia. DVM Thesis. Faculty of Veterinary Medicine, Addis Ababa University, Debre-Zeit, Ethiopia.
- Regassa A, Tassew A, Amenu K, Megersa B, Abuna F, et al. (2010) Across sectional study on bovine tuberculosis in Hawassa town and its surroundings, Southern Ethiopia. Trop Anim Health Pro 42: 915-920.
- Teklu A, Asseged B, Yimer E, Gebeyehu M, Woldesenbet Z (2004) Tuberculous lesions not detected by routine abattoir inspection: the experience of the Hossana municipal abattoir, Southern Ethiopia. Rev Sci Tech 23: 957-964.
- Shitaye JE, Getahun B, Alemayehu T, Skoric M, Treml F, et al. (2006) A prevalence study of bovine tuberculosis by using abattoir meat inspection and tuberculin skin testing data, histopathological and IS6110 PCR examination of tissues with tuberculous lesions in cattle in Ethiopia. Vet Med 51: 512-522.
- Radostits OM, Gay CC, Hinchelift KW, Constabel PD (2007) Veterinary Medicine. A text book of the disease of cattle, sheep, pig, goat and horses. (10thedn). Elsevier, London. pp. 1007-1040.
- OIE (1996) Diagnosis of bovine tuberculosis. In: Manual of standards for diagnostic tests and vaccines, pp. 267-271.
- O'Reilly LM, Daborn CJ (1995) The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle Lung Dis 76: 1-46.
- Radostits OM, Blood DC, Gay CC (1994) Diseases caused by Mycobacterium. In: Veterinary medicine, a text book of disease of cattle, sheep, pigs, goats and horses. 8th ed. London, Baillieretindu. Pp. 748-785.
- Omer M, Skjerve E, Woldehiwet Z, Holstad G (2001) Across sectional study of bovine tuberculosis in dairy farms in Asmara, Eritrea. Trop Anim Health Pro 33: 295-303.
- Phillips CJ, Foster CR, Morris PA, Teverson R (2003) The transmission of Mycobacterium bovis infection to cattle. Res Vet Sci 74: 1-15.
- Neill SD, Pollock JM, Bryson DB, Hanna J (1994) Pathogenesis of Mycobacterium bovis infection in cattle. Vet Microbiol 40: 41-52.
- Whipple LD, Boline AC, Miller MJ (1996) Distribution of lesion in cattle infected with mycobacterium bovis. J Vet Diagn Invest 8: 351-354.
- Tigre W, Alemayehu G, Abetu T, AmeniG (2012) Preliminary study on the epidemiology of bovine tuberculosis in Jimma town and its surroundings, Southwestern Ethiopia. Afr J Microbiol Res 6: 2591-2597.
- Müller B, Dürr S, Alonso S, Hattendorf J, Laisse CJ, et al. (2013) Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis 19.
- Neill SD, O’Brien JJ, Hanna J (1991) A mathematical model for Mycobacterium bovis excretion from tuberculous cattle. Vet Microbiol 28: 103-109.
- Nicholson MJ, Butterworth MH (1986) A guide to condition scoring of Zebu cattle. International Livestock Center for Africa (ILCA), Addis Ababa, Ethiopia, pp. 1-29.
- OIE (2009) Bovine Tuberculosis. OIE terrestrial manual. Paris, pp. 1-16.
- OIE (2010) Bovine tuberculosis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organization for Animal Health, pp: 683-698.
- Pal M (2013) Public health concern due to emerging and re-emerging zoonoses. Int J Lives Res 3: 56-62.
- Pal M, Zenebe N, Rahman MT (2014) Growing significance of Mycobacterium bovis in human and health. Microbes Health 3: 29-34.
- Russel DG (2003) Highlighting the parallels between human and animal tuberculosis. J Vet Med edu 30: 140-142.
- Shitaye JE, Tsegaye W, Pavlik I (2007) Bovine tuberculosis infection in animal and human populations in Ethiopia: a review. Vet Med 52: 317-332.
- Smith NH, Gordon S, Dela RR, Clifton-Hadley RS, Hewinson RG (2006) Bottlenecks and broomsticks : the molecular evolution of Mycobacterium bovis. Nat Rev Microbiol 4: 670-681.
- Thoen CO, Lobue PA, Enarson DA, Kaneene JB, De Kantor IN (2009) Tuberculosis: a re-emerging disease in animals and humans. Vet Ital 45: 135-181.
- Thrusfield MV (2007) Veterinary epidemiology. (3rdedn). Published by Black Well science Ltd. Edinburgh, UK. pp. 229-250.
- Torell R, Bruce B, Kvasnicka B, Conley K (2003) Methods of determining age of cattle. Cattle Producer's Library.
- Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, et al. (2002) Correlation of ESAT-6-specific gamma interferon with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun 70: 3026-3032.
- WHO (2003) Global tuberculosis control country profile. World Health Organization Report. pp. 99-101.
- WHO (2007) Tuberculosis: WHO fact sheet no. 104. Fact sheets on tuberculosis.
- Zeweld SW (2014) Cultural and molecular detection of zoonotic tuberculosis and its public health impacts in selected districts of Tigray region, Ethiopia. Sokoto J Vet Sci 12: 1-12.