Journal of Veterinary Science & Medicine
Download PDF
Review Article
Review on Triclabendazole Resistance in Fasciola
Warkaw Merachew1 and Tewodros Alemneh2*
1School of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
2Woreta City Office of Agriculture and Environmental Protection, South Gondar Zone, Amhara Regional State, Ethiopia
*Address for correspondence: Alemeneh T, Expert Veterinarian at Woreta City Office of Agriculture and Environmental Protection, South Gondar Zone, Amhara Regional State, Ethiopia, Tel: 251 9 20 49 98 20; Email: tedyshow@gmail.com
Submission: 05-August, 2020;
Accepted: 21-September, 2020;
Published: 25-September, 2020
Copyright: © 2020 Merachew W et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The control of Fasciolosis can be achieved by application of
anthelmintic drugs, elimination of the number of intermediate hosts
and reduction of exposure to infection. Triclabendazole, which is
a member of Benzimidazole, is most recommended and effective
way of controlling fasciolosis in animals and humans that can kill
both mature (adult) and immature liver flukes. This drug have able
to penetrate the tegument of Fasciola (F) hepatica by diffusion,
and the fluke is able to sulfoxidate the drug to the active sulfoxide
metabolite which binds to β-tubulin and thus inhibit the formation of
microtubules that are components of cytoskeleton of the parasite.
However, in recent year, resistance of Triclabendazole is reported in
animals and humans in different regions of the world. Resistance has
likely appeared due to a generally poor understanding of liver fluke
biology by farmers and con-founding factors, such as incorrect dosing,
inappropriate product choice, and lack of testing for efficacy. These
conditions may lead to reduced diffusion and metabolism of the drug,
change efflux pump activity and changes in the target molecule that
can reduce the effectiveness of Triclabendazole. Both in-vivo and
in-vitro methods, like Faecal Egg Count Reduction Test (FECRT) and
the Egg Hatch Assay (EHA), respectively, can help to investigate the
resistance of Triclabendazole. Administration of dual active flukicide
drugs, development of vaccines, implementation of Fasciola control
methods other than Triclabendazole, and use of accurate dosage at
appropriate time can help to reduce the incidence of Triclabendazole
resistance.
Keywords
Anthelmintics; Fasciola; Resistance; Triclabendazole
Introduction
Anthelmintics are drugs that are used to treat infections with
parasitic worms. This includes both flat worms, e.g., flukes and
tapeworms and round worms, i.e., nematodes. They are of huge
importance for human tropical medicine and for veterinary medicine.
Broad spectrum anthelmintics are effective against parasitic flat
worms and nematodes [1].
Triclabendazole (TCBZ), benzimidazole derivative, is one of the
major anthelminthic drugs used to control fasciolosis in domestic
animals. Triclabendazole was first introduced as a flukicide during
the early 1980s. It has an efficacious (> 98%) drug for both mature and
immature flukes and has been used to treat and control fasciolosis [2].
Due to its efficacy for immature flukes TCBZ is the best drug of choice
among other anthelminthic agents and considered as an Achilles heel
in the overall control of liver fluke [3]. This over-reliance on TCBZ to
treat sheep and, to a lesser extent, cattle, has resulted in selection for
flukes resistant to TCBZ [4]. The status of Triclabendazole-Resistance
(TCBZ-R) in F. hepatica has been reviewed elsewhere [5].
Benzimidazoles (BZs) are effective against a broad range of
parasites and also have wide safety margins, working at dosages of
mg/kg bodyweight [6]. Their mode of action appears to be mediated
through binding to β-tubulin within the parasite, thus inhibiting the
formation of microtubules that are central to the form and function
of the parasite’s cells. This prevents various essential cellular processes
such as the transport of secretory granules and enzymes in the cell
cytoplasm, resulting in cell lysis, with knock-on detrimental effects
on motility and feeding [7].
Resistance to Triclabendazole was first described in the United
Kingdom (UK) in the late 1990’s and has now been reported on
numerous occasions in fluke populations affecting sheep, and cattle.
Triclabendazole resistance is of interest, not only as part of the wider
trend of anthelmintic resistance, but also because its appearance
presents particular challenges to the management of ruminant
livestock, especially sheep, in many areas of the country. Resistance
has likely appeared due to a generally poor understanding of liver
fluke biology by farmers and con-founding factors, such as incorrect
dosing, inappropriate product choice, and lack of testing for efficacy
[8].
Mechanisms involved in the development of resistance to the
TCBZ can result from changes in the target molecule, in drug uptake/
efflux mechanisms and in drug metabolism [9]. Different methods,
both in vivo and in vitro methods, have been used to detect and
monitor Triclabendazole resistance. Faecal egg count reduction test
is the most used in vivo method and different in vitro methods are
described, example; the Egg Hatch Assay (EHA) [10].
A number of strategies have been proposed that may help to
avoid or at least slow down the development and spread of TCBZ-R.
They include limiting the number of treatments; strategic dosing at
particular times of the year, based on epidemiological data; correct
dosage; and the annual rotation of anthelmintic, using drugs from
different chemical groups. The latter strategy is designed to prevent
the build-up of resistance to a particular class of anthelmintic and to
minimize the passage of resistance genes early in the selection process.
However, a more effective approach is to use combinations of drugs.
It is particularly useful when development of resistance reduces the
efficacy of an individual drug, but it retains its efficacy in synergistic
combinations [11]. Therefore, the objectives of this work were to
review Triclabendazole resistance which is currently applicable for
the treatment of fasciolosis and to give highlights on the management
strategies to combat Triclabendazole drug resistance.
The Disease: Fasciolosis
Fasciolosis is among the important parasitic diseases in tropical and subtropical countries which limit productivity of ruminants in particular cattle. Fasciola hepatica and F. gigantica are the two liver flukes commonly reported to cause fascioliasis in ruminants [12]. Fasciola spp. infects mammals worldwide, mainly ruminants, but also humans can become infected. In ruminants, and especially
in sheep, the infection reduces feed conversion, growth, and meat and milk production. Moreover, it is one of the major causes of liver condemnations at abattoirs and interferes with fertility and fecundity. Fascioliasis is a disease that affects the liver parenchyma and bile ducts of numerous animals, including humans, which causes economic losses and threatens public health [13].
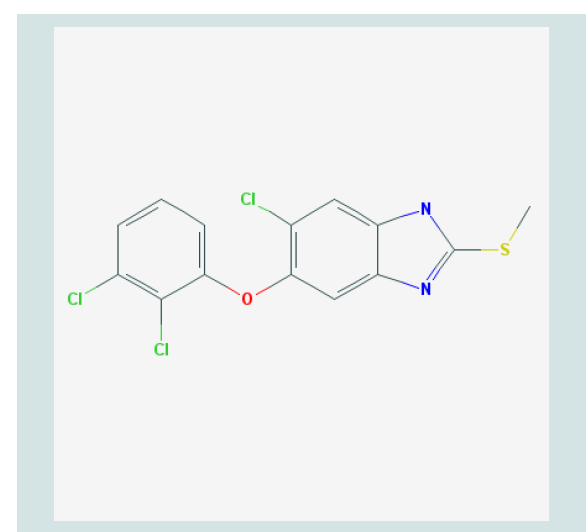
Figure 1: Molecular Structure of Triclabendazole [21].
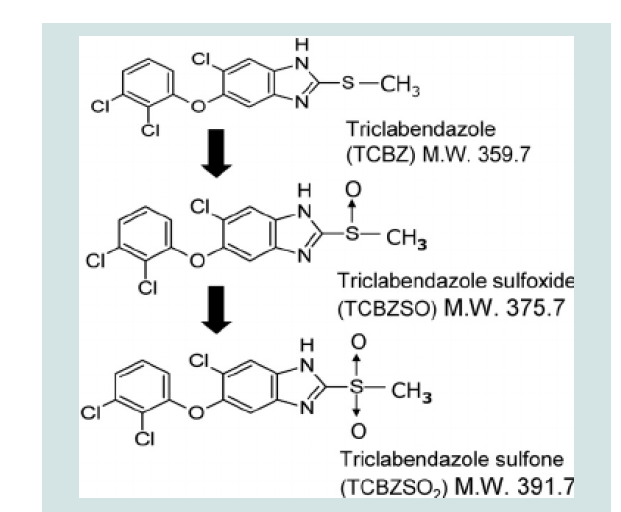
Figure 2: Chemical Structure of Triclabendazole and its Metabolites [23].
Control and Prevention of Fasciolosis
Control measures should be done on a preventative rather than
curative. Three effective control strategies have been used which are: using of anthelmintic to reduce the number of liver fluke in the
definitive hosts and the number of fluke eggs on the pastures, reduce
the number of intermediate host and reduce of exposure to infection
by managing the fluke prone areas [14].
Use of anthelminthics:
The correct time to use anthelmintics based on weather and
climate conditions. Drugs play a crucial role in the control of
fascioliasis. More frequent treatments are necessary if you use drugs
that are only effective against advanced mature flukes aged 12-16
weeks or older. Using Triclabendazole-based flukicides is the most
effective drug against both early mature and adult liver flukes. The
best control measures may be achieved if this drug use three times
yearly. August/September: to prevent pasture from contamination
and to eliminate adult flukes came from autumn and winter. January /
February: to completely remove of flukes picked up during late spring
and early summer. April/ May: to remove flukes picked up during
summer and early autumn [15].Snail control:
The second available strategy for control of Fasciola spp. is the
control of snail as it acts as an intermediate host for the parasite.
This can be done by; Chemical control: although chemical control
is effective, snails cannot be eradicated by chemicals because they
reproduce so readily. Improved drainage: Irrigation projects can
provide habitats to the snails. Cleaning of vegetation regularly may reduce the contamination of herbage [16].
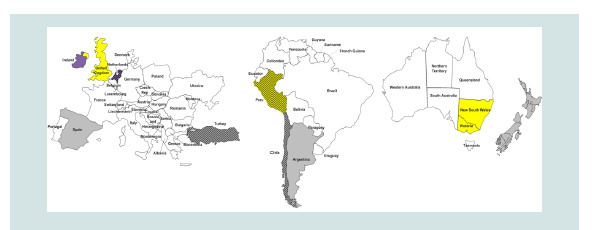
Figure 3: Description; Europe, South America, and Australia left to right,
respectively. Global Distribution of Reports of Triclabendazole Resistance
(TCBZ-R) in Livestock [34].
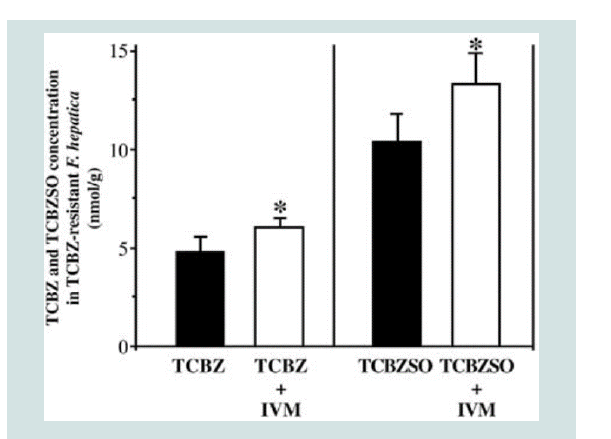
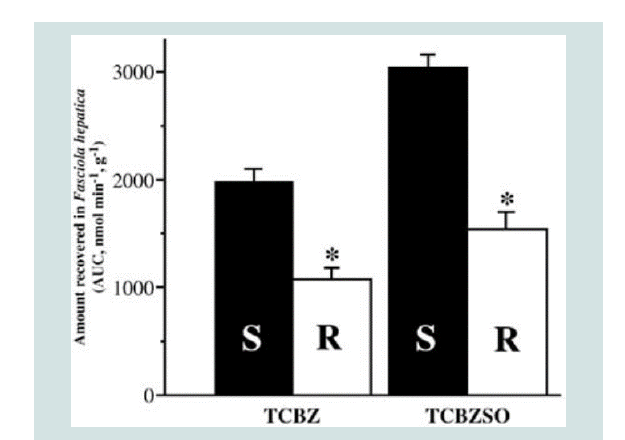
Figure 4: Decreased efflux of TCBZ and TCBZ.SO in TCBZ-resistant flukes
following co-incubation with Ivermectin. Where; * = P<0.05 [2].
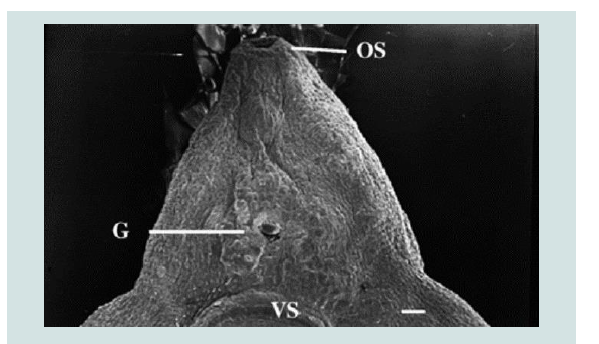
Figure 5: TCBZ-R fluke treated with TCBZSO (15 μg/ml) and verapamil (1×10−4 M) for 24 h. The surface tegument has been removed exposing the underlying basal lamina. OS: Oral sucker, G: gonopore, VS: ventral sucker [2].
Figure 6: Uptake of TCBZ and TCBZ.SO by TCBZ-S and TCBZ-R flukes. Where; * = P<0.05 [43].
Disease control by farm management:
This is the third effective strategy for control of Fasciola. This can
be accomplished by: Fencing the snail-infested grazing areas consist
only a small part of the animals’ pasture. Therefore, Fencing off
these contaminated areas would be the most economic and efficient
method of controlling fascioliasis. Spending a little money on fencing
may prevent a serious outbreak of liver fluke disease [17].The Drug: Triclabendazole
Effective strategies for the control of fasciolosis are mainly based
on the use of drugs. Triclabendazole (Fasinex®, Novartis) is worldwide
one of the most used drugs for the control of fasciolosis. TCBZ is
usually the anthelmintic of choice against F. hepatica in livestock, as
this drug has high activity against both adult and down to 1 week old
juvenile flukes [17]. In animals, TCBZ is the most effective and widely
used anthelmintic against immature and mature flukes [8]. Many
studies have been conducted on using of TCBZ showing high efficacy
against Fasciola spp. However, it has been revealed that in a later
study, the significantly low level of efficacies of TCBZ is the indication of resistance of F. hepatica against Triclabendazole in sheep [18].
Triclabendazole is the drug of choice in the treatment of
fascioliasis. However, in addition to the changing pattern of disease,
reports of resistance to TCBZ have appeared in the literature [19],
although they may not all represent genuine cases of resistance.
Nevertheless, any reports of resistance are a concern, because TCBZ
is the only drug that has shown high efficacy against the migratory
and juvenile stages of infection to date. Resistance to the drug could
potentially set back any recent gains made in the efforts to combat
and manage human and animal fascioliasis [20,21].
Triclabendazole is flukicidal BZs compounds extensively used in
veterinary medicine, and has excellent activity against mature and
immature stages of the liver fluke, F. hepatica. Triclabendazole is able
to penetrate the tegument of F. hepatica by diffusion, and the fluke is
able to sulfoxidate the drug to their sulfoxide metabolite (TCBZSO)
[22].
The results conducted by Mottier et al. indicated that the
tegument is an important target for TCBZ and albendazol action, and
also indicated that TCBZ is better than albendazole in all aspects of
the experiments. It could be concluded that TCBZ remains the drug
of choice for treating infection with the liver fluke, F. hepatica, and
also has become the main drug used to treat animals and human cases
[23,24].
Mechanism of action of triclabendazole:
To understand how resistance to TCBZ may develop, it is
necessary to understand the mechanism of drug action. TCBZ is a
BZs derivative and, by analogy with what is known about other BZs
drugs, it would be anticipated that TCBZ might bind to the β-tubulin
molecule and so disrupt microtubule-based processes. Evidence in
support of this idea has come from morphological studies on the
tegument, vitellaria and testis, following treatment with the active
sulphoxide metabolite. For example, there is inhibition of mitosis
in the vitelline and spermatogenic cells; disruption of transport
processes in the tegument (the outer layer of a trematode), which leads
to progressively severe damage of the tegmental surface, culminating
in the total loss of the tegument [17].Loss of tubulin immune-staining in the tegmental syncytium has
also been observed the results suggest that the microtubules have
disappeared which, in turn, would prevent the movement of secretory
bodies from the cell bodies to the tegmental surface. This process is
vital for the maintenance of the integrity of the surface membrane
and its disruption would explain the severe morphological changes
seen [25].
Despite years of research, the precise mode of action of TCBZ is
still unclear. TCBZ is a BZ derivative and all available evidence from
gastrointestinal round-worms indicates that BZ anthelmintics bind to
α and β-tubulins within the cells of the parasite, causing disruption of
vital processes, such as feeding and digestion. Several morphological
studies of the effects of TCBZ and its active metabolites on F. hepatica,
have examined the tegument, vitellaria, and testis of the fluke; all
three tissues showed significant signs of Ultra structural disruption,
consistent with inhibition of microtubule-based processes [26].
There is also a concurrent loss of tubulin immune-staining in the tegmental syncytium, further implicating an interaction with tubulin
as the primary mode of action of TCBZ. That said, this has not helped
inform our understanding of TCBZ-R, because TCBZ-resistant flukes
do not carry the F200Y/E198A or F167Y mutations in β-tubulin,
implicated in BZ resistance in nematodes, suggesting that alterations
to β-tubulin are not a key component of TCBZ-R [27].
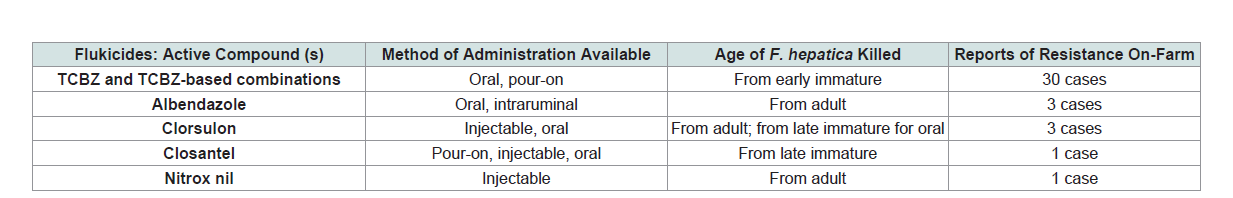
Table 1: List of currently available Triclabendazole products and other drugs used to control F. hepatica in cattle and sheep worldwide.
Recently, TCBZ was reported to inhibit adenylate cyclase activity
in yeast and/or inhibit the association of GTP-Ras with adenylate
cyclase. Most of the studies on the mechanism of action of TCBZ have
been carried out with TCBZ.SO. The precise mechanism remains to
be fully elucidated, but there is more evidence for an action against
microtubules and microtubule-based processes than for other
possibilities, such as against energy metabolism or neuromuscular
co-ordination [28].
New approaches to understand modes of action of triclabendazole:
The multiplicity of studies reporting different mechanisms of
resistance to TCBZ suggests that the mode of action of TCBZ and/or
the effects on fluke metabolism are complicated, but the advent of new
technologies could allow the target of TCBZ to be unraveled in the
foreseeable future. One approach is affinity purification of the putative
protein target, whereby TCBZ is immobilized to a solid support and
a protein extract is passed over the column, followed by elution of
any bound target proteins. This has resulted in the identification of
protein targets against several types of drug. However, these methods
seem best suited for situations where a high-affinity ligand binds a
relatively abundant target protein [29].A new approach to understanding the mode of action of small
molecules is the application of metabolomics, a whole-organism
assay approach that identifies metabolic perturbations in a cell
upon exposure to drugs. This technique identifies the metabolomics
compounds via mass spectrometry or nuclear magnetic resonance
and has been applied to several drug studies in various parasites. Thus,
a combination of approaches may be required to fully characterize
on-target and off-target effects of TCBZ and to clearly define the
mechanism(s) of TCBZ action [30].
Triclabendazole Resistance Distribution
TCBZ (FasinexTM) is the only commercial agent that kills young
pathogenic liver fluke, and is considered an Achilles heel in the
overall control of liver fluke. Unfortunately, suspected cases of liver
fluke parasites resistant to TCBZ have been reported, and without
intervention resistance is likely to establish as outbreaks of liver
fluke continue to spread [2]. Resistance to Triclabendazole was first
described in the UK in the late 1990’s and has now been reported
on numerous occasions in fluke populations affecting sheep. Exactly
how common TCBZ resistance is in different regions of the world
not known, but anecdotally it appears to be highly prevalent in fluke populations in sheep rearing areas [4].
Resistance of Triclabendazole described in different parts
of the world mostly in European countries such as Netherland,
Britain, Russia, Scotland and main land of Europe. The prevalence
of Triclabendazole resistance is high in these parts of the world it
may be due to more researches have been done in these countries.
In these countries Triclabendazole resistance examined by fecal egg
count reduction test, egg hatch assay, coproantigen reduction test
that indicates the presence of Triclabendazole resistance in those
countries [17].
In Britain, there are fewer reports of resistance to TCBZ in fluke
populations in cattle, which may reflect the less intensive use of TCBZ
in cattle. However resistance was described in 2010 in Scottish beef
calves and is becoming more evident as awareness increases. It is
important that farmers are warned of the risk of buying in animals
carrying resistant fluke populations and take appropriate advice about
quarantining animals particularly if coming from fluke endemic parts
of the country [31].
In mainland Europe, most reports of TCBZ-R have come from
the lower-lying northwestern countries, such as the Netherlands [8].
There are few, if any, reports of confirmed TCBZ-R from central or
southern Europe. This most likely reflects the general prevalence of
fluke and the perceived need to treat. There is a growing gradient
in the prevalence of F. hepatica west-to-east and south-to-north
in Europe, with prevailing climatic and/or underlying geological
conditions probably pivotal. Fox et al. predicted that fluke incidence
will increase and spread west-to-east in the UK over the coming
decades, based on modeling the Ollerenshaw Indices and UK Climate
Projections. Similar trends are predicted to occur across Europe.
The implication of this spread of liver fluke is of serious concern in
relation to TCBZ-R, since farmers in traditionally fluke-free regions
will need to treat animals that may have been exposed to TCBZresistant
flukes [32,33].
Risk Factors for triclabendazole resistance:
Resistance has likely appeared due to a generally poor
understanding of liver fluke biology by farmers and con-founding
factors, such as incorrect dosing, inappropriate product choice,
and lack of testing for efficacy. The high frequency of TCBZ use,
effectively TCBZ mono therapy with no anthelmintic rotation, was
a major contributing factor towards the development of TCBZ-R [8].
Since TCBZ is not a persistent chemical, resistance was likely due to
head selection in contrast to tail selection observed with roundworms
[34,35].The failure of TCBZ to kill liver fluke could be due to several
factors ranging from problematic drug delivery, reduced host liver
metabolism of TCBZ to active pro-drug, or management practices that select for TCBZ resistant parasites. The inability of the FECRT
to indicate why the drug has failed means that veterinarians cannot
fully advise on the spectrum of potential solutions. Thus, current
advice if egg counts fail to fall after TCBZ treatment is to switch to an
alternative but less effective drug and recommend that TCBZ dosing
is suspended to eliminate threat of ‘resistant’ parasites causing greater
production losses [36].
Human cases of triclabendazole resistance:
In recent years, fascioliasis has emerged as a major zoonotic
disease, with an increase in the number of human cases, and it is a
serious health problem in a number of countries. TCBZ is also the
drug of choice for treating fasciolosis in humans and it is conceivable
that TCBZ-resistant fluke populations, selected in livestock, could
pose a zoonotic risk to human health, especially in areas such as Peru
and Bolivia, where there is a high incidence of human infections [37].
The first incidence of TCBZ treatment failure in humans was reported
in a livestock farmer in the Netherlands, with further recent reports
of four cases from Chile, one case from Turkey, and seven cases
from Peru. Clearly, TCBZ-resistant zoonotic infections are a serious
emerging issue [38].Economic impact of triclabendazole resistance:
The economic significance of Triclabendazole resistance is that
increasing morbidity and mortality of the animal in addition the
capital loss due to the treatment and control of Triclabendazole
resistance of fasciolosis. There is also high economic and zoonotic
effect of Triclabendazole resistance Fasciola strain if the strain is
transmitted to human. An estimate of the total cost of the outbreak of
fasciolosis that was compounded by the presence of Triclabendazoleresistant
F. hepatica was, therefore, approximately £19,200. This
figure corresponds to £8.73 per ewe, and does not include additional
labor costs that were incurred [39].Modes of Triclabendzole Resistance
Investigations into the mechanism of resistance to TCBZ have
used the Sligo isolate of F. hepatica. This isolate has been shown to be
resistant to the action of TCBZ in vivo, at both the adult and juvenile
stages. Flukes from this isolate also resist the action of TCBZ.SO in vitro, even at abnormally high concentrations [25].
Mechanisms involved in the development of resistance to the
TCBZ can result from changes in the target molecule, in drug uptake/
efflux mechanisms and in drug metabolism [9]. With regard to
changes in the target molecule, the target is presumed to be β-tubulin,
but tubulin staining is not abolished by TCBZSO in the resistant
isolate. However, in nematodes Benzimidazole resistance has been
linked to selection of a β-tubulin isotype with a phenylalanine to
tyrosine substitution at position 167 or at position 200. Some amino
acid differences have been noted at other positions but whether these
amino acid changes are relevant to the resistant phenotype or are due
to normal allelic variation in the genes encoding this isotype remains
to be determined and many more sequences from individual TCBZsusceptible
(TCBZ-S) and -resistant (TCBZ-R) flukes will need to be
obtained [40].
Studies are underway in both adult and juvenile fluke to identify
the drug-sensitive isotypes by localizing the sites of expression of the various α- and β-tubulin isotypes, and thus determining which
isotypes are expressed in areas that are severely disrupted following
TCBZ treatment. At the molecular level, structural studies have
shown that the residues that are variable in benzimidazole-resistant
organisms are brought together to form a cluster during the folding
of the β-tubulin protein. These also indicated that the cluster of
“sensitive” residues was not on the surface of the molecule, raising
the question of “how could the drug access this region? [41].
Molecular modeling studies using β-tubulin sequences from the
liver fluke and the nematode Haemonchus contortus have been used
to propose a solution. By analogy to the bacterial tubulin homologue
FtsZ the angle between the N-terminal, intermediate and C-terminal
domains of β-tubulin was relaxed by 11°. This increased the surface
area of the potential benzimidazole binding cleft sufficiently for
Triclabendazole to be “docked” in this region. Mammalian and liver
fluke tubulins presented a smaller region for binding, commensurate
with the restricted effects of Benz imidazole in these organisms [42].
It was proposed that the resistance-conferring mutations at residues
200 and 167 were effective as they allowed the formation of hydrogen
bonds “closing off” the binding pocket. The model also suggests
that benzimidazoles act not by causing the de-polymerization of
microtubules, but by locking the β- tubulin moieties in the “open”
conformation and thus interfering with the formation of heterodimers
with α-tubulins prior to microtubule formation. The entry of TCBZ
into the fluke has been shown to occur mainly by diffusion across the
tegmental syncytium rather than by oral ingestion [24].
The diffusion of both TCBZ and TCBZ.SO into TCBZ-R (Sligo)
flukes is significantly lower than in TCBZ-S (Cullompton) flukes
[43]. Interestingly, this is not true for the related BZ, albendazole
whose uptake is similar in both TCBZ-S and TCBZ-R fluke. The
results suggest that the mechanism is specific to TCBZ and that
P-glycoprotein-linked drug efflux pumps could potentially be
involved in the resistance mechanism. Overexpression of Pgp
has been linked to resistance in nematodes to different classes of
anthelmintics. Experiments with Pgp inhibitors have shown that it
is possible to “reverse” the condition of the flukes, from resistant to
susceptible. For example, co-incubation with Ivermectin decreased
the efflux of TCBZ and TCBZ.SO in TCBZ-R flukes such that the
drug was present at levels comparable to those in TCBZ-S flukes [44].
In contrast, Ivermectin had no impact on the uptake of albendazole
in either TCBZ-S or -R flukes. The consequence of Pgp inhibition in
TCBZ-R fluke has been demonstrated in a separate morphological
study with another Pgp inhibitor, R (+) -verapamil. Co-incubation
of R (+) -verapamil plus TCBZ.SO led to severe disruption of the
tegument of TCBZ-R flukes, whereas treatment with TCBZ.SO on
its own (even at a high concentration) caused minimal changes to
the tegmental surface. The disruption to the resistant fluke was
comparable to that observed in susceptible flukes following treatment
with TCBZ.SO. While a change in efflux pump activity may simply
represent a nonspecific mechanism, nevertheless, it is likely to play a
significant role in the development of resistance [17].
The identification and localization of the Pgp-linked efflux pumps
have yet to be determined. Studies using a laser micro dissection
protocol have provided small quantities of specific fluke tissues for
Pgp localization. Tegument, gut and reproductive structures have been isolated and probed with a Pgp specific primer. The results
obtained to date are inconclusive and many more specimens need
to be examined. With regard to a role for altered drug metabolism
in TCBZ resistance, the sulphoxidation of TCBZ to TCBZ.SO and
TCBZSO to the sulphone metabolite (TCBZ.SO2) are both greater in
TCBZ-R than -S flukes [45].
Indeed, TCBZ-R flukes have a 39% greater capacity to metabolize
the parent drug. Use of inhibitors has shown that the flavinmonooxygenase
(FMO) enzyme system is the main pathway for the
metabolism of TCBZ, and it is more important than the cytochrome
P450 enzyme system. Moreover, methimazole (MTZ, an FMO
inhibitor) had a significantly greater inhibitory impact on TCBZ
sulphoxidation in TCBZ-R than -S flukes (43% as against 34%). By
comparison, the cytochrome P450 inhibitor, piperonyl butoxide
reduced TCBZSO formation to a lesser extent and the inhibition was
equal (at 12%) in the two isolates [43].
Detection of Triclabendazole Resistance:
Different methods, both in vivo and in vitro methods, have been
used to detect and monitor AR. Faecal egg count reduction test is the
most used in vivo method and gives an estimation of the efficacy of
the drug by comparing the egg counts pre and post treatment. The
accuracy of the method depends on a correlation between egg counts
and worm burdens which is not always present. Different in vitro
methods are described. The EHA was first described by Le Jambre for
the detection of BZ-resistance. Modification of the original method
is developed by Taylor et al. and the method is mostly used for the
detection of possible BZ resistance in sheep and horses [46].In-Vitro method:
The detection of resistance to Triclabendazole (TCBZ) in sheep
infected by F. hepatica was studied using an EHA. Fasciola hepatica
eggs were recovered from bile and faeces of infected animals by
isolates with different grade of anthelmintic resistance to TCBZ: i)
a resistant isolate (RT); ii) a susceptible isolate (ST); iii) naturally
infected sheep by a susceptible field strain (FST). The EHA is based on
the ovicidal properties of some BZs, and on the capacity of eggs from
resistant isolates to embrionate and hatch at higher concentrations
than those ones from a susceptible isolate [47]. Although the EHA
was originally designed to detect AR in Gastrointestinal Nematodes
(GIN), some studies have been carried out with F. hepatica eggs from
gall bladder and/or faeces using TCBZ, Albendazole (ABZ) and their
sulphoxide metabolites [48].A commercial formulation of TCBZ (Fasinex®) diluted in
Dimethyl Sulfoxide (DMSO) was used to carry out the EHAs. The
concentration of TCBZ in this commercial formulation was 50 mg/
ml. Dilutions of 10, 40, 200, 1000 and 5000 μg/ml were prepared to
obtain a final concentration in the wells of 0.05, 0.2, 1, 5, and 25 μg/
ml after adding 10 μl of each dilution to a total volume of 2 ml. In
all EHAs, control wells with 10 μl of DMSO were included. Eggs
from faeces were obtained by sedimentation, from animals infected
by ST and from a pool of faeces of sheep naturally infected by FST.
Fasciola hepatica eggs were directly recovered from the gall bladder
and washed several times with tap water by sedimentation [47].
In-Vivo method:
The main method used to identify TCBZ-R in the field has
been the Faecal Egg Count Reduction Test (FECRT), with the
recommended post-treatment sample collection time point at 21 days
[49]. Other studies using experimental infections have used 14 days
for post-treatment sample collection, which may not allow sufficient
time for all eggs from dead parasites to pass out of the gall bladder
and be excreted [50].The FECRT is probably most often used, with drug treatment
being regarded as successful if there is a 95% reduction in fluke egg
counts by 14 days post-treatment. However, it is known that eggs can
be stored in the gall bladder for several weeks, so they may still be
present, even though the flukes have been successfully removed; this
can lead to false positive results. Moreover, egg production by flukes
ceases within 2 days of successful TCBZ treatment [51].
Other disadvantages of the test include the fact that there is
no standard method (i.e. sedimentation, floatation, individual or
composite samples) and faecal egg counts are not related to fluke
numbers; also, for diagnosis of infection, it only detects patent
infections and egg shedding is irregular. Fluke counts may be more
accurate but are not always carried out and this data runs into problems
of trial design and how the flukes are recovered. The FECRT is often
used for field cases, though it suffers from the problems outlined
above and is not always linked to fluke count data. Controlled clinical
trials should be, but are not necessarily always, carried out [52].
Management Strategies to Delay Development of Triclabendazole Resistance
Use of other drugs and their combinations:
The only chemical options for the control of TCBZ-resistant
fluke are, depending on the host species, treatment with clorsulon,
nitroxynil, closantel, albendazole, or oxyclozanide [53]. The use of
dual-active flukicides has been recommended to control a F. hepatica
isolate that was resistant to Triclabendazole and clorsulon when these
drugs were administered individually; this isolate was susceptible
to these drugs when given as a dual-active formulation. When such
formulations have a synergistic effect (i.e., have greater efficacy than
the sum of the actives), this may increase the lifespan of the respective
actives. Synergy has been seen with several dual-active flukicides (e.g.,
TCBZ+ clorsulon or TCBZ+ luxabendazole) against TCBZ-resistant
fluke in sheep [54].Vaccines:
An alternative approach to control TCBZ-R would be the
development of a livestock vaccine for F. hepatica, which would
reduce fluke burdens irrespective of the drug-resistance status of the
flukes and would not compromise fluke control during lactation.
However, no commercial liver fluke vaccine exists, although several
experimental vaccines for livestock are under development. No
vaccine has shown reproducibly high enough efficacies (> 60%)
in cattle to warrant commercial production, although the leucine
aminopeptidase (LAP) vaccine has shown high efficacy (up to 89%)
in sheep [55].Thus, until a new anthelmintic is developed that kills all
developmental stages, including the early immature fluke, a vaccine
is the only alternative treatment that could provide ongoing control of fluke infections in livestock in regions where TCBZ-R is endemic
[56].
Integrated Parasite Management for Farms:
The management practices on farms generally rely solely upon
anthelmintics and appear to have contributed to the development
of resistance. Management practices must change to preserve the
longevity of existing flukicides, because the likelihood of any new
flukicides coming to market in the near future is low [55].Throughout the year, there are periods in which the risk of fluke
infection is higher and these periods fluctuate depending upon
location and prevailing climatic conditions, but do provide a set of
guidelines to determine when treatment may be required. If farmers
combine strategic treatments with FECs and the cELISA during
high-risk periods, this approach could be used to determine when to
drench, which drench to use, or whether treatment is required at all,
based on the known thresholds for economic loss [57].
Well-executed strategic treatments will minimize the need
for further treatments throughout the year and, therefore, help to
preserve the efficacy of existing flukicides. Regular drug efficacy
testing, using FECRT and/or CRT, to preserve the efficacy of existing
flukicides or TCBZ is essential to allow producers to avoid using
products with reduced efficacy and prevent economic losses resulting
from unidentified resistance [55].
Flukicides should always be administered according to the
product specifications and best-practice methods, which include:
weighing individual animals or the heaviest in the herd to determine
dose, calibrating drench equipment before use and during treatments,
selecting the most potent formulations of product, and, where
possible, regularly rotating effective products. In addition, we must
also look at how pastures, drinking water, and irrigation can be better
managed to decrease the likelihood of F. hepatica infection. Pasture
management can allocate low-risk pastures (such as newly sown
paddocks, hay, or silage paddocks) to young animals during the highrisk
periods, to limit the chances of parasite transmission [58].
Conclusion
In conclusion, livestock production has a great potential to
rural farmers in the world. It can be well exploited if fasciolosis
and Triclabendazole drug resistance are controlled very well.
Triclabendazole drugs are the most realistic means to control animal
fasciolosis. However, the increasing trends of Triclabendazole use and
Triclabendazole resistance are a serious problem to cattle production
in the world. Since there will no new products become available in
the near future, it is of utmost important to maintain the efficacy of
Triclabendazole. The widespread incidence of TCBZ-R in livestock
will be a major threat to global livestock production and producers
need see alternative treatments, such as new flukicides or vaccines
to control infections. Based on the above conclusion, the following
recommendations are forwarded; strict supervision on the usage of
Triclabendazole drugs should be implemented; professionals and
livestock owners should be well aware of about Triclabendazole drug
and its resistance; more attention should be given to the adoption of
integrated parasite management strategies in the farms to control the
parasite; since there is no literature available on Triclabendazole in Ethiopia, more researches ought to be done regarding Triclabendazole
resistance and its efficacy in various parts of the country.
Acknowledgement
Authors would like to express their deepest thanks to God
for his permission to do all daily activities as well. Authors also
would like to say thanks to Mr. Sileshi Belew for his excellent
guidance and advice, valuable simulative suggestions, necessitated
encouragements and overall supervisions that highly aid for the
completion of this work.