Journal of Veterinary Science & Medicine
Download PDF
Research Article
Optimization of Chitin Extraction, Physicochemical and Functional Properties of Chitosan Production from Shells of Karamote Shrimp Peneaus (Melicertus) Kerathurus in Western Greece
Katsoulis K1* and Rovoli M2
1Department of Animal Husbandry and Nutrition, University of
Thessaly, Greece
2Biochemistry Department, University of Thessaly, Greece
*Address for Correspondence: Katsoulis K, Assistant Professor, Department of Animal Husbandry and Nutrition, University of Thessaly, Faculty of Veterinary Science, 224 Trikalon Street, P.O. Box 199, Karditsa, 43100, Greece; E-mail: kkatsoulis@uth.gr
Submission: 12-December, 2020;
Accepted: 25-January, 2021;
Published: 28-January, 2021
Copyright: © 2021 Katsoulis K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This research aims to optimize by chemical methods the extraction of chitin and chitosan from shells of Karamote shrimp Peneaus (Melicertus) kerathurus. Shrimp waste can be used as source of high value compounds. Chitin is a major component of the exoskeleton
of invertebrates and chemically is a linear polysaccharide of β (1→4)
linked N-acetylglucosamine monomers. Chitosan is a deacetylated
form of chitin having d-glucosamine repeating units linked by β (1-4)
glycosidic bond. The extraction method uses different concentrations
of sodium or potassium hydroxide in the deproteinization (protein
separation) and deacetylation (remove acetyl groups) step and
hydrochloric acid for demineralization (separation of calcium
carbonate and calcium phosphate) to yield optimum output. Among
all experiments, results of 1.0 N solution of HCl for demineralization, 2
N for deproteination and 12.5 N NaOH solutions for deacetylation at
solid to solvent ratio of 1: 15, clearly demonstrate a significant yield of
chitin and chitosan. The results obtained from this study show also that
the solubility of chitosan in 1% acetic acid solution achieved up to 90%.
Keywords:
Fishery byproducts; Chitin; Chitosan; Shrimp shell; Deacetylation
Introduction
In recent years great interest has been expressed in isolating
components using by-products and wastes. Fish wastes include
byproducts or many fish species having no or low commercial
value, undersized or damaged commercial species. Large amounts
of culture wastes are associated with the environmental impact
on aquatic ecosystems, since the release of organic wastes might
significantly change the community structure and biodiversity of the
benthic assemblages [1,2]. The objective of reducing fishery discards
and to avoid environmental problems can be achieved by establishing
alternative solutions, such as technologies to enhance and transform
fish wastes as an economic resource, for example by developing
techniques of extraction and concentration of the bioactive compounds
they contain. Crustaceans, belonging to the Decapoda order, include
prawns, shrimps, lobsters, crayfish and crabs. Melicerthus kerathurus
known as karamote prawn is one of the above group. It is a demersal
crustacean, widely distributed inhabiting the Mediterranean Sea
and the eastern Atlantic from the south coast of England to Angola
where it lives on soft bottoms of the continental shelf, less than 60
m depth [3]. The shell of crustaceans consists of 20-30% chitin, 30-40% protein and 30-50% calcium carbonate and calcium phosphate,
and other minor constituents, such as lipids, astaxanthin and other minerals [4]. Chitin and chitosan are β (1-4) glycans whose chains are
formed by 2-acetamide-2-deoxy-D-glucopyranose and 2-amino-2-
deoxy-D-glucopyranose units, respectively. Chitin is the second most
abundant polysaccharide on earth, following the cellulose. Chitin can
be obtained from the cell wall of fungi, the exoskeleton of arthropods,
the shells of mollusks and the beaks of cephalopods including
cuttlefish, octopuses and squids. Chitin is presented mainly in three
allomorphs: α-chitin, with antiparallel chains, is the most abundant
and it is isolated from the exoskeleton of crustaceans, particularly
from shrimps and crabs; β-chitin, with parallel chains, is presented in
the cell walls of diatoms and in the skeletal structures of cephalopods,
and commonly extracted from squid pens; γ-chitin is presented in
fungi and yeast, which is a combination of the α and β allomorphs
[5]. Chitosan is generally prepared by the deacetylation of chitin with
alkali. Due to their useful biological properties (biocompatibility,
biodegradability, antimicrobial activity) and chemical modification
potentials because of their reactive functional groups (-OH, -NH2,
and -COOH) both have been used in a wide range of fields including
biomedical, food production, and wastewater treatment fields [6-8].
The current research was performed to evaluate the suitable acid and
alcali concentration for extraction of high-quality chitin and chitosan
from shells of Melicertus kerathurus.
Materials and Methods
Description of the fishing area: The experimental fishery of the shrimps was carried out twice
in October 2019 in Kalamos channel, along the Western Greek
coast of the Ionian Sea, near the Kalamos Island (Lat.38.61581o
Ν, Log.20.90102o Ε). Administratively, the island belongs to the
Prefecture of Lefkada Island covering an area of 25 km2.
Raw material: A total number of 234 specimens were collected. Firstly, the
shells were removed from the animal and secondly the specimens
were packed in polyethylene bags, placed on ice, transported to the
laboratory and were stored in a freezer at -20 °C until further use.
Reagents: All the chemicals and solvents used were purchased from Sigma-
Aldrich at the analytical grade or highest level of purity available and
used as received. A commercial chitosan with a deacetylation degree
of 75% was chosen. All solutions were freshly prepared in distilled
Methods
Laboratory sample preparation was needful to convert the
shell sample into a homogeneous material suitable for analysis.
Before grinding, the biggest parts of shell samples were crushed and
divided in smaller. Drying of samples was obtained by heating in a
drying oven (model R. Espinar, S.L.) at 100-103 °C until constant
weight was obtained between two sequential measurements [9,10].
Drying samples grinded in a mill (System POLYMIX® PX-MFC
90 D) into smaller particles using sieve with 2 mm wide openings.
pH measurements were made using a digital laboratory pH meter
(model WTW pH 525) which was calibrated using certified pH= 4.0
and pH= 7.0 buffer solutions, according to the official method [9].
The Ether Extract (EE) was determined using method of Soxhlet.
Approximately 2000 mg of solid sample were mixed with anhydrous
sodium sulfate, placed in an extraction thimble and were extracted
using an appropriate solvent in the Soxhlet extractor. The distilled
solvent was condensed and in final drying step the remaining traces
of solvent was evaporated from the boiling flask. The mass of the
extract (total fat) was measured after subtracting initial from final
weight of the boiling flask. Ash contents were determined using dry
ashing method. The samples (2000 mg) were ashed for about 8 hr.
until a white or grey ash residue had been obtained using a furnace (model P. Selecta, 3000 W) where temperature had been gradually
increased from room temperature to 450 °C in 1 h [9,10]. The
solubility of chitosan was carried in dilute solution of acetic acid. 1000
mg of chitosan obtained from the deacetylation process was dissolved
in 100 mL of 1% acetic acid solution and stirred by magnetic stirrer
until a homogeneous solution was obtained. The chitosan acidic
solution was then filtered using a vacuum pump. The procedure was
repeated three times. The insoluble content was calculated from the
weight of insoluble particles obtained on the filter and the weight of
chitosan dissolved. The water binding capacity (wbc) was calculated as
follows; 10 ml of distilled water with 1000 mg of chitosan was mixed
on a vortex for 15 min and centrifuged at 3500 rpm for 30 min. After
centrifugation, supernatant water was poured off and the sample was
weighed. WBC (%) = [Bound water (g)/Initial chitosan weight (g)]
* 100. The oil binding capacity (obc) was calculated as follows; 10 ml
of sunflower with 1000 mg of chitosan was mixed on a vortex for 15
min and centrifuged at 3500 rpm for 30 min. After centrifugation,
supernatant oil was poured off and the sample was weighed. OBC (%)
= [Bound oil (g) / Initial chitosan weight (g)] * 100 [10].
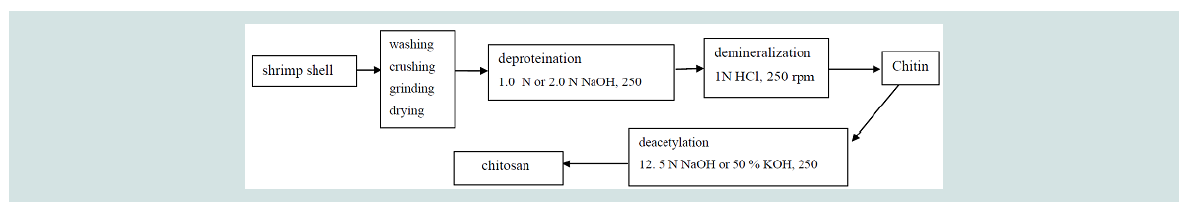
Extraction of chitin by chemical method: Deproteination (Dp): A total of 5-20 g dry samples of raw
shrimp shell waste were treated with 1.0 N and 2.0 N NaOH at solid
to solvent ratio 1:5, 1:15, 1:18 and 1:20 (w/v), with constant stirring at
200 rpm for 24 hours at room temperature, with pH ranged from 11-13. After that, the solution was filtered and the samples were washed
with distilled water to neutrality in running tap water. Water from
the samples was removed before performing the demineralization
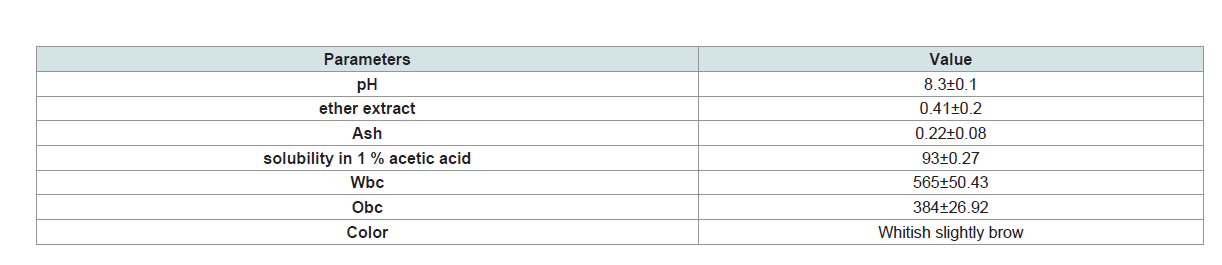
Table 3: Physicochemical and functional properties of chitosan (values are expressed as Mean± S.D (n=5).
Demineralization (Dm): Samples from deproteination process
were treated with 1.0 N HCl in the ratio 1:10 (w/v), with constant
stirring at 200 rpm for 24 hours with pH value ranged pH 1.0-2.5
at room temperature. After that, the solution was filtered and the
samples were washed with distilled water to remove acid and calcium
chloride. The samples were then dried for 3 hours using an oven at
80 oC until constant weight was obtained. The dried sample is now
known as chitin.
Chitosan production:
Deacetylation (Da): The deacetylation process was conducted
by soaking dried chitin prepared from demineralization in a 12.5 N
solution NaOH and 50% (w/v) solution KOH with constant stirring
at 200 rpm for 24 hours at room temperature. After that, the product
is known as chitosan. Chitosan was washed with tap water until
neutral (pH 6.5-8.0) and dried as described in deproteination and
demineralization.Chitin and chitosan yield:
The percentage of the yield of chitin was calculated by dividing the weight of extracted chitin to initial dry shrimp shell weight.Yield was calculated as follows: Yield of chitin (%) = (extracted
chitin, g)/shrimp shells, g) * 100
The percentage of the yield of chitosan in relation to chitin was
calculated by dividing the weight of produced chitosan to dry chitin
before deacetylation.
Yield was calculated as follows: Yield of chitosan (%) = (produced
chitosan, g)/chitin, g) * 100
The percentage of the final yield of chitosan was calculated by
dividing the weight of produced chitosan to initial dry shrimp shell
weight.
Results
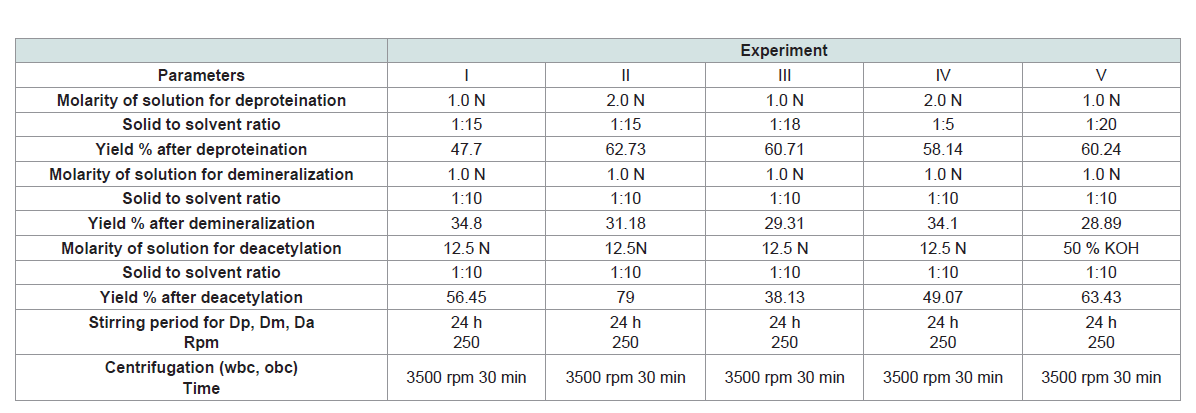
Parameters and details of experiments are demonstrated in
(Table 1). Some differences can be observed attributed to the changes
of molarity of solutions (NaOH, KOH, HCl) for deproteination,
demineralization and deacetylation and to the ratio of solid to solvent. As a result, the percentage of yield differs in all experiments.
The higher values of yield after deproteination were observed in exp
II (62.73) and III (60.71) while the lowest value was observed in exp
I. Also, the percentage of yield after demineralization (as a ratio to
previous step of deproteination), varied from 29% to 35%. The higher
values of yield after demineralization were observed in exp I (34.8),
IV (34.1) and II (31.18) while the lowest value was observed in exp
V (28.89). The percentage of yield after deacetylation (as a ratio to
previous step of demineralization), varied from 38% to 79%. The
higher values of yield after deacetylation were observed in exp II (79)
and V (63.43) while the lowest value was observed in exp III (38.13).
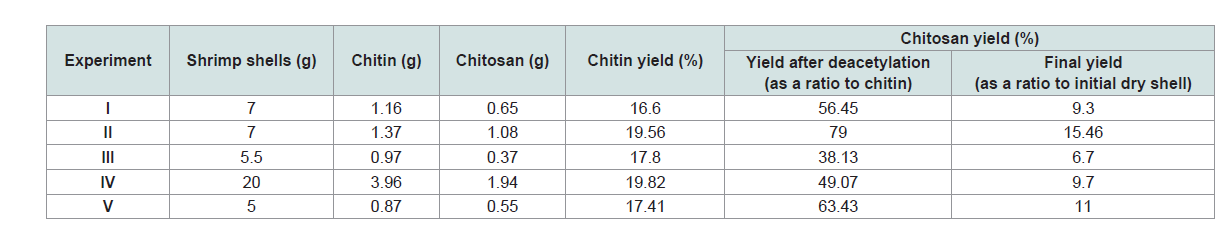
The percentage of yield of chitin and chitosan was presented
in (Table 2). Differences can be observed in the experiments. The
percentage of yield of chitin varied from 16.6 to 19.82, the higher
values were observed in exp II (19.56) and IV (19.82) while the lower
value in exp I (16.6). Also, the percentage of yield of chitosan varied
from 9.3 to 15.46, the higher value was observed in exp II (15.46) and
V (11) while the lower value in exp III (6.7).
Functional and physicochemical properties of chitosan that have
been studied in this work have shown a variety of characteristics as it
is demonstrated in (Table 3). The color of chitosan is whitish slightly
brown while the percentages of water binding capacity and oil
binding capacity are 565 and 384, respectively. Chitosan shows high
solubility in 1% acetic acid (93%) while values of ether extract and ash
are below of 0.5%.
Discussion
Results from this work clearly demonstrate a variety of the
percentage yield of chitin and chitosan. These values note the
importance of the treatments of deproteination, demineralization
and deacetylation and can be attributed to the differences of molarity
of solutions (NaOH, KOH, HCl) and to the ratio of solid to solvent.
Concerning the parameters of the experiments and the percentage
yield it is believed that the extraction process can be improved to gain
higher yields of chitin and chitosan [11-15]. In our experiment results
of 1.0 N solution of HCl for demineralization, 2 N for deproteination
and 12.5 N NaOH solutions for deacetylation at a solid to solvent ratio
of 1:15, clearly demonstrate a significant yield of chitin and chitosan.
Recovery of chitosan in the present study is similar to chitosan yield
(15%) from shrimp shell waste reported by and slightly higher than
yield of chitosan (12%) in study of [16,17].
Functional and physicochemical properties of chitosan indicate
a good quality product with valuable properties[18]. Solubility is
an important property to determine the quality of chitosan; high
solubility refers to a good quality chitosan. Chitosan is soluble in
dilute organic acids, like acetic acid or formic acid and insoluble in
water and in basic pH solutions. Its solubility depends on distribution
of N-acetyl and free amino groups. Chitosan is protonated because of
the presence of amino group in the aqueous acid solution which leads
to its solubility [19]. Also, higher values of solubility are combined
with increasing degree of deacetylation due to removal of acetyl group
from chitin [20]. In our experiments, chitosan showed high solubility
in 1% acetic acid (93%) and its whitish slightly brown color is similar
to color of chitosan of other studies [21]. The higher values of water
and oil binding capacity of chitosan compared with reported studies found to be within the range. Finally, the lower values of the ash and
ether extract content prove the purity of the sample indicating the
completion of demineralization and deproteinization and confirm
the quality of chitosan [22].
Conclusion
In the procedure developed in the present study, chitosan was
obtained as a white slightly brown powder with sufficient functional
physicochemical properties. Improving extraction processes by
changing experiment parameters the yield percentage of chitin and
chitosan could be increased giving an alternative solution in seafood
industries to obtain valuable products.