Journal of Veterinary Science & Medicine
Download PDF
Research Article
Investigation of Antiviral Effect of Far-UVC Microplasma Lamp against Influenza A Virus (H9N2)
Do HQ1, Park YH2, Kim SS3, Lee J4, Jung WK2* and Chung HC5,6*
1Department of Veterinary Medicine Virology Lab, College of
Veterinary Medicine and Research Institute for Veterinary Science,
Seoul National University GwanAk-Ro 1, GwanAk-Gu, Seoul 151-
742, South Korea
2NoAH Biotech Co., Ltd., Suwon 16614, South Korea
3NANOCMS Co., Ltd., Cheonan 31040, South Korea
4Computational Neurobiology Laboratory, Salk Institute of
Biological Sciences, La Jolla, CA 92037
5Department of Microbiology and Immunology, Institute for
Immunology and Immunological Diseases, Brain Korea 21 PLUS
Project for Medical Science, Yonsei University College of Medicine,
Seoul 03722, South Korea
6Department of General Medicine, International European
University, Nieszawska 19, 61-021 Poznań, Poland
*Address for correspondence:
Jung WK, NoAH Biotech Co., Ltd., Suwon 16614, South Korea; Email:
wkj@noah-biotech.com; Phone: +82-31-292-1257
Chung HC, Department of Microbiology and Immunology, Institute for
Immunology and Immunological Diseases, Brain Korea 21 PLUS Project
for Medical Science, Yonsei University College of Medicine, Seoul
03722, South Korea & Department of General Medicine, International
European University, Nieszawska 19, 61-021 Poznań, Poland; E-mail:
heeskyi@yuhs.ac; Phone: +82-2-2228-1836
Submission: 15 September, 2022
Accepted: 17 October, 2022
Published: 19 October, 2022
Copyright: © 2022 Do HQ, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Influenza A virus is one of the most serious diseases in the world.
Therefore, it is necessary to find an effective and safe method to prevent the
spread of the disease. A far-UVC at 222nm is considered safe and effective
for viral and bacterial treatment. In this study, virucidal effects and the safety
status of far-UVC microplasma were evaluated in vitro against influenza
A virus H9N2 0130 strain. The results (from TCID50 and real-time PCR)
indicated that a far-UVC inhibited influenza A virus depending on dosage.
A far-UVC eliminated 99.99% of the virus at doses of 44 and 56 mJ/cm2 in
clarified and un-clarified solutions, respectively. Moreover, a far-UVC 222 nm
did not have any harmful effects in MDCK cell at dose 78 mJ/cm2. Our study
provided useful information in a far-UVC application against influenza A virus.
Keywords
Influenza A virus (H9N2); Microplasma lamp; Far-UVC (222nm);
Inhibition
Introduction
Influenza A virus (IAV), an enveloped virus with segmented,
negative single-strand RNA linear genome, is one of the most serious
pathogens in the world, causing significantly negative impacts on
economy and human-animal health (Epstein & Price, 2009). Based
on its surface antigens, IAV was classified into different subtypes
related to the antigenic characteristics of hemagglutinin (HA) and
neuraminidase [1]. To date, 18 types of HA (H1 - H18) and 11 type
of NA (N1 - N11) were identified [2], among which the last two HA
and NA subtypes tended to be specific to bats [3]. Subtypes H1N1
and H3N2 currently spread throughout the human population [4-7]. Similarly, H5, H7, and H9 still cause serious problem in poultry
production as evidenced by high mortality rate and loss of egg
production.
Due to their rapid rate of contagion, it is difficult to effectively
control IAV and other air-borne diseases. Disinfectant agents
might be harmful for human health, possibly causing eye and skin
irritation, and may result in damage to the equipment surfaces such
as discoloration in textiles due to corrosive metals [8]. Ultraviolet
light at wavelength of 254 nm or above, which is also widely applied
to prevent diseases, may cause skin cancer and cataracts [9]. Recently,
the application of far-UVC light at wavelength range of 200 - 230
nm as a potential disinfection method has been the interest of
many studies [10]. This type of UV was demonstrated to effectively
inactivate a numerous of pathogens including bacteria and viruses
[5,10]. This type of far-UVC is also considered safe for humans [8].
However, not many studies focused on controlling the spread of IAV.
In this study, we investigated the virucidal effects against H9N2 as an
IAV subtype model using a microplasma far-UVC lamp, primarily
emitting a wavelength of 222 nm.
Material & Methods
A microplasma lamp (UV222050 x 050, Eden Park Illumination,
Inc., Champain, IL, USA) with the emission wavelength of 222 nm
was applied and the UV irradiation fixture and setup were designed
and prepared (NANOCMS Co., Ltd., Cheonan, Korea) to have an adjustable distance between the target sample surface and light source.
The UV exposure conditions were well described in the previous
study [9]. IAV serotype H9N2 01310 vaccine strain and MDCK
cell line were kindly provided by Professor Kang Suk Choi (Avian
laboratory, College of Veterinary Medicine, Seoul Nation University).
Virus solution was spread in Petri dishes (60 mm) and the irradiation
time was varied from 10 seconds (1.3 mJ/cm2) to 10 minutes (78 mJ/
cm2). Treated virus and non-treated control were serially diluted in
maintain media (DMEM plus 1 μg/ml TPCK-treated trypsin and 1%
NEAA) and inoculated in to MDCK cell cultured in 96-wells plate.
After 1 hour of adsorption, the cells were carefully washed three
times, replaced by 100 μl of fresh maintain media and incubated at
37oC, 5% CO2 for 5 days. The cells were observed daily to detect the
presence of cytopathic effects and TCID50 was calculated using the
Reed and Muench method [11,12]. Each condition was tested three
times.
The presence of genetic trace of IAV was also examined by
quantitative RT-PCR. Viral RNA was extracted from treated solution
using RNA extraction kit (Intron Biotech, Korea) according to
the manufacturer’s protocol. RNA was converted to cDNA using
SuperScript III First-strand synthesis kit (Invitrogen, USA). Real-time
PCR was performed using Maxima Sybr green/Rox qPCR master mix
(ThermoFisher, USA) using specific primers (Table 1).
Cytotoxic analysis of far UVC irradiation was performed using
MTT assay. In brief, 5 x 104 MDCK cell were seeded into each well of
the 96 wells plate and incubated at 37oC, 5% CO2 overnight. Cell was
irradiated with far UVC light for 10 minutes. The viability of cells was
evaluated using CyQUANT™ MTT Cell Viability Assay (Invitrogen,
USA) according to manufacturer’s instruction.
Statistical analysis was performed using GraphPad Prism 8.0
(GraphPad Software Inc., USA). Virus titer in each treated condition
was compared using one-way ANOVA and Turkey analysis. The
inhibition growth curve was calculated using nonlinear regression
curve analysis.
Results & Discussion
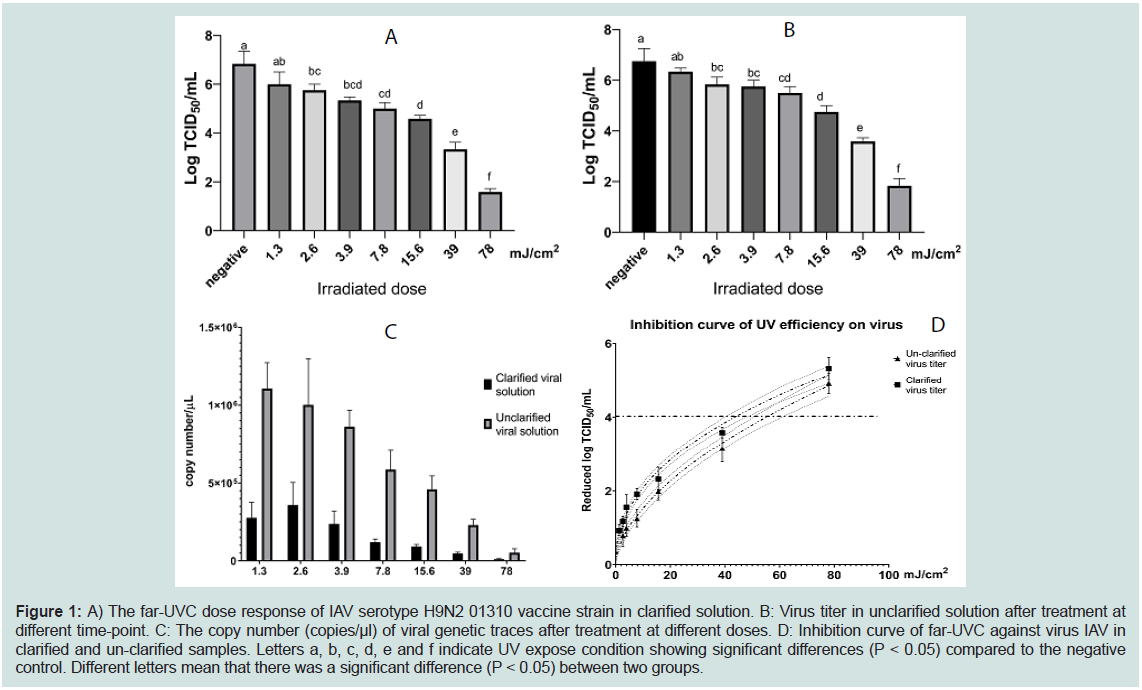
First, we investigated the effect of far-UVC microplasma on
clarified virus. The results indicated that, far-UVC (222nm) inhibited
AIV serotype H9N2 01310 vaccine strain in a dose-dependent
manner. Specifically, a dose of 2.6 mJ/cm2 significantly reduced
the viral titer when compared to the untreated condition (Figure 1A). Additionally, 78 mJ/cm2 exposure doses (corresponding to 10
minutes of treatment) inhibited almost all viruses in the experimental
condition (Figure 1A). Moreover, to answer the question about the
effect of cell debris on virucidal activity of far-UVC, we performed a
similar experiment with un-clarified virus solution. Similar trend of virus inhibition was also noticed in this experiment (Figure 1B). 2.6
mJ/cm2 irradiated dose decreased the virus titer by approximately 0.8
log10 TCID50 while UVC irradiated at 78 mJ/cm2 caused a reduction
of virus titer to 1.8 log10 TCID50 (Figure 1B). These results were
supported by the reduction of viral RNA trace (Figure 1C).
The effective irradiation doses, which was defined as the treatment
condition that reduced virus by 4 log10 TCID50 was calculated based
on the dose-inhibition curve as described in the method section. The
result indicated that the effective irradiation doses were approximate
44 and 56 mJ/cm2 in clarified and un-clarified solution, respectively.
Therefore, far-UVC microplasma irradiation was slightly more
effective against clarified virus than cell-debris containing fluid
(Figure 1D).
Figure 1: A) The far-UVC dose response of IAV serotype H9N2 01310 vaccine strain in clarified solution. B: Virus titer in unclarified solution after treatment at
different time-point. C: The copy number (copies/μl) of viral genetic traces after treatment at different doses. D: Inhibition curve of far-UVC against virus IAV in
clarified and un-clarified samples. Letters a, b, c, d, e and f indicate UV expose condition showing significant differences (P < 0.05) compared to the negative
control. Different letters mean that there was a significant difference (P < 0.05) between two groups.
Previous study indicated that irradiation dosage at 7.8 mJ/
cm2 eliminated almost all SARS-CoV-2 in solution (Jung et al.,
2021). Moreover, Buonanno, Welch, Shuryak, and Brenner (2020)
demonstrated that lose dose at 1.7 and 1.2 mJ/cm2 can remove
99.9% of alpha HCoV-229E and beta HCoV-OC43 in aerosol [3].
For IAV, 222 nm UVC at 2mJ/cm2 can inactivate more than 95%
of aerosolized H1N1 [14-17]. However, in our study, irradiated
doses at approximately 23 mJ/cm2 and 33 mJ/cm2 were necessary to
inactivated 99.9% H9N2 virus in clarified and unclarified solutions,
respectively. The higher effective dose in this study might be due to its
wavelength that penetrated less into the liquid solution. In this study,
comparing with the clarified sample, cell-debrid containing sample
need a higher dose of irradiation. This result could be explained by
the fact that cell-debrid might absorb the UV energy, resulted in
decrease the virucidal efficiency. Ma et al. also suggested the effect of media component on the virus sensitivity to UV exposure [10].
Nevertheless, this study provided useful evidence of antiviral activity
of far-UVC light in aqueous solution.
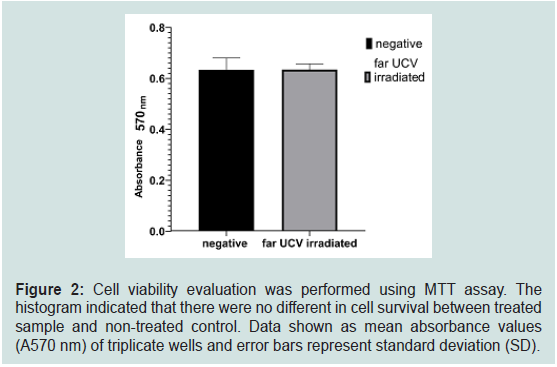
In our study, 10 minutes treatment effectively reduced the
infectivity of IAV serotype H9N2 01310 vaccine strain. Therefore, we
continuously examined the cytotoxic effect of this condition under
in-vitro experimental conditions. MTT assay revealed that 78 mJ/cm2
irradiated dose did not cause harmful interference against MDCK
cell line under experimental condition (Figure 2). A far-UVC 222 nm
wavelength light was considered safe for humans. In detail, long-term
exposure to far-UVC microplasma at 222 nm wavelength could not
induce cancer in the sensitive model experiment [18,19]. Similarly,
Fukui et al. (2020) indicated that UVC with wavelength of 222 nm at
a dose of 500 mJ/cm2 only slightly induced DNA damage in skin after
treatment [7]. In our study, there were no differences in cell survival
in exposed experimental and non-exposed control, indicating the
safety of 222 nm far- UVC at a dose of 78 mJ/cm2 in vitro.
Figure 2: Cell viability evaluation was performed using MTT assay. The
histogram indicated that there were no different in cell survival between treated
sample and non-treated control. Data shown as mean absorbance values
(A570 nm) of triplicate wells and error bars represent standard deviation (SD).
Conclusion
In conclusion, this study demonstrated that far UVC microplasma
irradiation effectively removes the infectivity of influenza virus
without harming the cell. Our results suggested the effectiveness and
safety of far UVC microplasma irradiated dose.
Funding Source
This work was supported by the Korea Science and Engineering
Foundation (KOSEF) grant funded by the Korea government (No.
2020R1I1A1A01054539).