Journal of Veterinary Science & Medicine
Download PDF
Case Report
Diagnostic Investigation of a Tembusu Virus Infection in Broiler Breeders
You Wang*1, Peiyong Li1, Shanping Cao1, Baomin Duan1, Aijian Qin2, Kun Qian2 and Huaguang Lu1,3*
1Tianjin Ringpu Biotechnology Co., Ltd., Dongli District, No.1-the
9th E. Road, Tianjin 300000, China
2Department of Preventive Veterinary Medicine, College of Veterinary Medicine, Yangzhou University, No.12 East Wenhui Road, Yangzhou 225009, China
3Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA 16802, USA
2Department of Preventive Veterinary Medicine, College of Veterinary Medicine, Yangzhou University, No.12 East Wenhui Road, Yangzhou 225009, China
3Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA 16802, USA
*Address for correspondence:Huaguang Lu, Department of Veterinary and Biomedical Sciences,
The Pennsylvania State University, University Park, PA 16802, USA.E-mail Id: hxl15@psu.edu
Submission:16 October, 2024
Accepted:30 October, 2024
Published:05 November, 2024
Copyright: © 2024 Wang Y, et al. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Keywords:Tambusu Virus; Broiler Breeders; Virus Isolation; Virus
Detection; Sequencing
Abstract
This report describes our diagnostic findings on isolation and
characterization of a Tambusu virus (TMUV) infection in a broiler
breeder flock, which caused a sharp decline in egg production and
primary clinical symptoms of lethargy, reduced appetite and watery
diarrhea. The main autopsy lesions observed in affected breeder
chickens were reproductive system abnormalities, including follicular
membrane hemorrhage and follicular liquefaction. This TMUV infection
resulted in about 1.8% mortality in eight weeks. The TMUV isolation was
made from oviduct specimens and conducted in both embryonated
chicken eggs and chicken embryo fibroblast cells cultures. Amino
acid analysis of the virus structure of envelope protein (E protein)
indicated that the TMUV isolate belonged to the same branch of duck
TMUV vaccine strains of FX2010-180P, DF2 and HB, which were widely
used in domestic ducks in China. Notably, the chicken TMUV isolate
exhibited the highest homology (99.0%) with the TMUV DF2 and HB
vaccine strains. The isolation and characterization of chicken-derived
TMUV in this study brings an urgent need for further investigations into
the impact of TMUV infections on egg-type chickens to enhance
prevention and control strategies for better performance of egglaying
hens of commercial layer farms and boiler breeder flocks.
Introduction
Tembusu virus (TMUV) belongs to the Ntaya virus group of
flaviviruses within the Flaviviridae family. It is a single-stranded RNA
virus with a genome length of 10,990 bp. The genome encompasses
an open reading frame (ORF) that encodes three structural proteins
(capsid protein C, PrM protein, and envelope E protein) alongside
seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B,
and NS5). The E protein is the largest structural component of TMUV
and acts as the primary virulence antigen. The E protein contains
multiple antigenic determinants crucial for viral processes, including
adsorption, replication, and biosynthesis [1].
The TMUV was initially detected in ducks in China in 2010 [2].
TMUV infections in ducks cause notable decline in egg production due
to primary affecting and damaging the target organs of reproductive
system characterized by ovarian hemorrhage, follicular rupture, and
follicular membrane hemorrhage. Subsequently, TMUV infections
have been diagnosed in chickens, geese, sparrows, and mice [3-5]. In
this study, we report a TMUV infection occurred in broiler breeder
chickens and findings of isolation and characterization of the TMUV
strain to provide new evidence of TMUV infection in chickens.
Materials and Methods
Disease Onset, Sample Collection and Preparation:
A disease onset with a sharp decline in egg production occurred in
a large-scale broiler breeder flock with over 53,000 broiler breeders at
34-weeks of age, which continued for a period of 8 weeks and caused
about 1.8% mortality. Six dead birds during the second week of disease
onset were submitted to our laboratory for diagnostic tests. The
autopsy examinations showed all the six birds had prominent lesions
of follicular membrane hemorrhage and follicular liquefaction; but
no observable pathologic lesions were seen in other organs. Tissue
specimens of the follicular membranes were collected for laboratory
diagnostic tests of possible viral pathogens. Other tissue samples of
trachea, lung, liver and spleen were collected for screening tests of
avian viruses commonly infected poultry in the region. The same type
of tissue specimens from the six birds were pooled and minced with
sterile scissors and homogenized at 1:5 dilution (w/v) with sterile
phosphate buffered saline (PBS, 8mM NaH2PO4, 150mM NaCl, 3mM
KCl, and 2mM KH2PO4 at pH 7.4). The homogenate underwent three
freeze-thaw cycles of freezing at-80°C and thaw at 37°C temperatures,
followed by centrifugation at 12,000 rpm for 10 min. The resulting
supernatant was filtered through a 0.22 μm syringe filter and stored at
-80 °C freezer for various diagnostic tests in this study.Virus isolation (VI) in embryonating chicken eggs (ECE) and cell cultures:
Specific-pathogen-free (SPF) chicken fertile eggs, which were
obtained from Jinan Sais Poultry Technology Co., Ltd (Pingan
Nanqiao District, Jinan, Shandong Province, China), were incubated
at 37°C egg incubator in our laboratory for avian VI. When the ECE
reached 9-10 days of age, the prepared tissue specimens were each
inoculated into 5 ECE via allantoic cavity rote, 0.2 mL per egg. The
specimen-inoculated ECE were incubated at 37°C egg incubator
and candled daily for 5 days. If embryos perished, they were
removed and placed in a 4°C refrigerator. After 5 d incubation, all
ECE were removed and placed at 4°C refrigerator for a minimum
5 h or overnight, then allantoic fluid (AF) samples were harvested
and embryos were examined. Embryo specimens were collected if
specific embryo lesions were observed. The AF samples were used for
inoculation of next egg passage for three consecutive passages.The specimen of follicular membranes was also conducted for
VI in UMNSAH/DF-1 cell line (ATCC, CRL-3586). The DF-1 cell
cultures were prepared in 25 cm2 cell culture flasks for specimen
inoculation when the DF-1 monolayer cells reached about 80% or
>80% fluency. Briefly, 1) discard the cell culture medium; 2) add
about 2 mL sterile PBS to the flask to wash the monolayer cells
gently and then discard the PBS; 3) inoculate 0.5 mL of the filtered
specimen homogenate into the 25 cm2 flask; 4) incubate the flask in
a 37°C incubator for 40 min; 5) add 4.5 mL cell culture maintenance
medium to the flask. The specimen-inoculated cell culture flask was
incubated in a 37°C incubator with 5% CO2 supplement and was
examined daily for a period of 5 days. If cytopathic effects (CPE) were
observed and developed about 70% or >70% of the monolayer cells,
the CPE positive cell flask was placed in -80°C freezer for harvest. If
no CPE were observed at 5 d pi, the cell culture flask was removed
from incubator and placed in -80C for harvest. After froze-thaw 3
times, the flask cell culture material was transferred to a 15 mL
centrifuge tube and centrifugated at 1200 rpm for 10 min, and then
the supernatant was used for inoculation to next cell passage for a
total of three serial cell passages.
PCR and One-Step RT-PCR for Virus Detection:
VI samples of AF and cell culture materials were processed for
detection of avian influenza virus (AIV), Newcastle disease virus
(NDV), infectious bronchitis virus (IBV), egg-dropping syndrome
virus (EDSV), TMUV, and Mycoplasma Synovia (MS) by PCR and
One-Step RT-PCR. DNA and RNA extractions were conducted using
a fully automated nucleic acid extractor and its automatic nucleic acid
extraction kit (the extractor Model No. VNP-96P, Nanjing Vazyme
Biotech Co., Nanjing, China) in accordance with the manufacturer’s
instructions. PCR for MS and EDSV, RT-PCR for NDV, IBV,
AIV H9 and TMUV were performed. The resulting products were
electrophoresed on a 1% agarose gel, and gel imaging was conducted
after electrophoresis for result observation and recording.Primers sequences for NDV [6], IBV [7], AIV H9 [7], EDSV [8],MS [9], and TMUV [10] were referred from publications
[Table 1]and were synthesized by Sangon Biotech Shanghai Co., Ltd. (698 Xiangmin Road, Songjiang District, Shanghai, China). One-Step RT-PCR kit, Taq Master Mix, and TAE electrophoresis buffer were
obtained from TransGen Biotech (Catalog No. AT411-02, Zhongguan-
cun Dongsheng International Science Park, Haidian District,
Beijing, China). The PCR instrument of T100 thermal cycler and gel
electrophoresis instruments (PowerPac universal power gel imager
ChemiDoc MP) were acquired from Bio-Rad Laboratories (Shanghai)
Co., Ltd., Pudong, Shanghai.
The PCR program included pre-denaturation at 95°C for 5 min;
denaturation at 95°C for 1 min, annealing at 60°C for 15 s, extension
at 72°C for 1.5 min for 32 cycles; and a final extension at 72°C for 10
min.
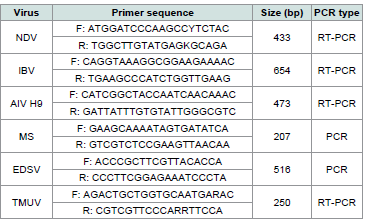
Table 1:Sequences of PCR and RT-PCR primers, target genes and fragment
sizes for detection of NDV, IBV, AIV-H9, MS, EDSV and TMUV.
The One-Step RT-PCR program involved reverse transcription at
50°C for 30 min, pre-denaturation at 95°C for 3 min; denaturation at
95°C for 30 s, annealing at 55°C for 35 s, and extension at 72°C for 1.5
min for 32 cycles; with a final extension at 72°C for 10 min.
Cloning and sequencing analysis of E protein gene of TMUV:
RNA samples were extracted from the positive CPE cell culture
material, which was tested positive for TMUV, the PCR products of
target bands were recovered, ligated with a pMD18-T vector, and
transformed into DH5α receptor cells. Positive colonies were selected,
verified by colony PCR [11], and submitted to Sangon Biotech for
sequencing. The MegAlign program[12] was used for analyzing and
comparing sequencing results. The MEGA7 software was used for
construction of evolutionary tree graph. Sequence information of 24
TMUV reference strains retrieved from GenBank was provided in
[Table 2].Results
Clinical symptoms and gross pathologic lesions:
The disease onset caused nearly 30% decline in egg production
and about 1.8% mortality for a period of 8 weeks. Observations of
clinical symptoms included a sudden decline in egg production,
lethargy, reduced appetite, and watery diarrhea with green and
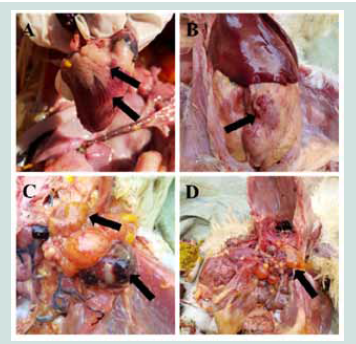
white feces. Autopsy examinations revealed gross pathologic lesions
of myocardial congestion, coronary fat hemorrhage, abdominal
fat hemorrhage, follicular membrane hemorrhage, and follicular
liquefaction[Figure 1].Isolation of TMUV:
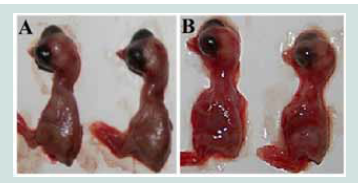
The follicular membrane specimen-inoculated ECE exhibited
mortality in 48-120 h pi. Dead chicken embryos displayed edema
and hemorrhage on the embryo body [Figure 2] and chorioallantoic
membrane. The follicular membrane specimen-inoculated DF-1 cell
cultures exhibited CPE lesions of increased refractivity, rounding,
and fusion at 48 h pi. As cell death and detachment occurred,
many translucent, round cells appeared in the culture medium. The
follicular membrane specimen generated VI samples of AF, embryo
homogenate and cell culture materials were tested all positive for
TMUV, but negative for NDV, IBV and AIV by RT-PCR; and negative
for EDSV and MS by PCR. Embryo lesions were not observed on the
ECEs inoculated with other tissue specimens, and the harvested AF
samples were negative by the PCR/RT-PCR screening tests.TMUV E protein gene homology and evolutionary tree analysis:
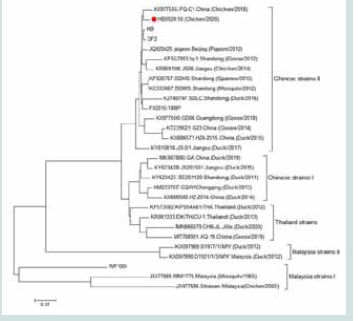
This TMUV strain isolated from broiler breeder chickens was
named HB202010. Amino acid homology analysis revealed that the
HB202010 strain demonstrated a homology ranging from 94.4% to
99.0% with TMUV reference strains. The lowest homology was seen
Figure 1: Autopsy lesions in the morbid broiler breeder flock. A: Cardiac
congestion and coronary fat hemorrhage; B: Abdominal fat hemorrhage; C:
Follicular membrane hemorrhage; D: follicular liquefaction.
with the live vaccine strain WF100, while the highest homology was
seen with the inactivated vaccine strains DF2 and HB. Evolutionary
tree analysis positioned the HB202010 strain within the same branch
as the live vaccine strain FX2010-180P and inactivated vaccine strains
DF2 and HB. All these strains belonged to the Chinese TMUV strains
II. Notably, this HB202010 strain did not fall within the same branch
as the live vaccine strain WF100 [Figure 3].
Discussion
The newly emerged flavivirus or TMUV infectious disease
occurred and spread rapidly in domestic ducks in the East and North
regions of China since April 2010. Research studies indicated that
TMUV caused a sudden decline in egg production and pathogenic
lesions in follicular membranes and folliclesin egg-laying ducks [2,12-14]. TMUV transmission is unlike the traditional flaviviruses to
be primarily transmitted by insect vectors, TMUV remains prevalent
even during fall and winter when mosquito populations are scarce.
In 2018, researchers in Shanghai Veterinary Research Institute
reported that some epidemic strains gained airborne transmission
Figure 2:Hemorrhage lesions of SPF chicken embryos in 120 h pi with
TMUV positive specimens. A: Control group; B: Virus inoculation group.
due to a mutation at amino acid position 156 [15]. This discovery
confirmed that TMUV transmission is not solely dependent on insect
vector of mosquitoes. In terms of pathogenicity, the TMUV strains,
which were isolated originally from mosquitoes in Kuala Lumpur of
Malaysiain 1955, exhibited low pathogenicity to poultry in Southeast
Asia countries [16]. However, its pathogenicity has significantly
increased since the TMUV endemic outbreaks in ducks in China in
2010. TMUV infections in ducks caused severe pathologic lesions of
hemorrhage of follicular membranes, follicular deformation, atrophy,
and liquefaction, resulting in a sharp decline in egg production and
substantial economic losses in the duck industry. In regard to host
spectrum, ducks were the primary host of TMUV infections, but by
2012, research findings indicated that TMUV infections occurred in
other poultry species of chickens and geese [3,17]. Subsequent studies
showed TMUV infections in sparrows, suggesting their potential role
as vectors [4]. In 2013, TMUV was found to be able to infect mice,
posing a public health risk [5]. Additionally, a study in the same
year showed high seropositivity among workers in TMUV-infected
duck farms, with 71.9% of workers being seropositive for TMUV
antibodies and 47.7% of throat swabs being positive for the virus [18].
In the present study, the TMUV field isolate from broiler breeder
chickens caused symptoms of depression, loss of appetite, and a
sharp decline of egg production. Autopsy findings showed follicular
membrane hemorrhage and follicular liquefaction. Screening tests
were negative for other pathogens commonly affecting egg-laying
hens, such as IBV, EDSV, and MS. Thus, screening for TMUV
should be considered when a layer flock experienced declines in egg
production, particularly adjacent to duck farms. TMUV infections
in broiler breeders could occur between early and peak egg-laying
stages. Manifestations associated with TMUV infections include a
slowly increasing or not reaching peak in egg production, or a short
peak followed by a substantial 15-30% drop of egg production for
2-3 weeks. In general, our observations on the TMUV affected flocks
showed that observable clinical symptoms of TMUV infections could
be 4-5 weeks or longer up to 8 weeks within an affected flock, and the
spread of TMUV infections to adjacent flocks or poultry premises is
relatively slow. The mortality rate could be around 0.1% ~ 0.3% daily
in about two weeks of the active infection period.
Laboratory diagnosis for TMUV cases is commonly conducted
by VI using ECE or embryonating duck eggs (EDE). The specimen inoculated
ECE or EDE should develop pathologic lesions of the
embryos, such as hemorrhage and edema on the entire embryo,
edema of the chorionic allantoic membrane, hemorrhage or enlarge/
swollen on embryo organs of liver and kidneys [2,12-14]. The
pathogenicity of duck origin TMUV strains was documented to
intensify following around100 consecutive passages in EDE, while
its potency weakens after 100 passages in ECE [19]. Research studies
demonstrated TMUV strains possess proliferation capabilities in
various avian cell lines (DF-1, LMH, CEF, DEF) and mammalian cell
lines (Vero, HEK293T, HCT116, A549) [19]. In our present study,
DF-1 cells showed distinct cellular lesions in 48 h pi, contributing
valuable insights into TMUV pathogenicity and cellular interactions
within an avian context.
The E protein, a fundamental surface structural protein of
TMUV, serves as the primary antigen for eliciting the production
of neutralizing antibodies. Existing evidence underscores the
multifaceted role of the E protein, not only mediating viral adsorption
to host cells but also facilitating fusion with the host cell membrane
and active participation in the invasion process [20]. Consequently,
the gene encoding the E protein is conventionally utilized as the
principal reference gene for evolutionary analyses of TMUV. In a
comprehensive investigation, Yu, et al conducted an evolutionary
tree analysis encompassing the open reading frame (ORF), E, NS1,
NS3, and NS5 genes of 78 representative TMUV strains originating
from Southeast Asia and mainland China [21]. The outcomes of this
analysis delineated five major branches, denoted as Malaysian (1955),
Malaysian (2012), Thai (2013), and Chinese isolate I and Chinese
isolate II. Notably, the Chinese isolate II branch emerged as the
prevailing and dominant lineage in China. In alignment with these
findings, the evolutionary tree analysis in our study was focused on
gene encoding of the E protein, positioning isolate HB202010 within
the Chinese isolate II branch. The amino acid homology observed
between isolate HB202010 and other reference strains within the
same branch ranged from 97.6% to 99.0%, underscoring the limited
degree of variation exhibited by this isolate.
Considering the absence of effective pharmaceutical interventions
for treating TMUV infection, vaccine immunization stands out as the
foremost strategy for impeding TMUV transmission, complemented
by robust biosecurity measures. Currently, there are commercially
available inactivated vaccines for HB and DF2 strains, and live
vaccines for WF100 and FX2010-180P strains. These vaccines have
played a pivotal role in shielding ducks from TMUV infection,
effecting a transformation in TMUV epidemiology from widespread
outbreaks to localized occurrences. Notably, the HB202010 isolate
from our present study aligns with the same lineage as the vaccine
strains HB, DF2, and FX2010-180P. Given the escalating reports of
TMUV-infected chickens in recent years, we advocate for proactive
vaccine immunization in breeder farms at elevated risk, administered
prior to the onset of laying eggs. This approach ensures that antibody
titters during the laying stage remain effective in countering TMUV
infection.