Journal of Forensic Investigation
Download PDF
Research Article
*Address for Correspondence: David Grubb, Department of Clinical Sciences, Faculty of Medicine, Lund University, Department of Anaesthesia and Intensive Care, Skane University Hospital; SE-221 85 Lund, Sweden, Tel: +46 46 17 82 24; Fax: +46 46 17 82 51; E-mail: david.grubb@med.lu.se
Citation: Grubb D, Frigyesi A, Finnhult M, Dencker D, Olsson SG, Lindberg L. Breath Alcohol Analysis by Standardization to Water Vapour Enables Contact Free Sampling with Preserved High Accuracy and Precision As Compared With Mouthpiece Sampling. J Forensic Investigation. 2014;2(1): 6.
Copyright © 2014 Grubb D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Forensic Investigation | ISSN: 2330-0396 | Volume: 2, Issue: 1
Submission: 31 January, 2014| Accepted: 17 April, 2014 | Published: 19 April, 2014
Breath Alcohol Analysis by Standardization to Water Vapour Enables Contact Free Sampling with Preserved High Accuracy and Precision As Compared With Mouthpiece Sampling
David Grubb1*, Attila Frigyesi1, Mikael Finnhult2,Daniel Dencker2, Sven-Gunnar Olsson2 and Lars Lindberg1
- 1Department of Clinical Sciences, Faculty of Medicine, Lund University, Department of Anaesthesia and Intensive Care, Skane University Hospital; SE-221 85 Lund, Sweden
- 2Servotek AB, Arlöv, Sweden
*Address for Correspondence: David Grubb, Department of Clinical Sciences, Faculty of Medicine, Lund University, Department of Anaesthesia and Intensive Care, Skane University Hospital; SE-221 85 Lund, Sweden, Tel: +46 46 17 82 24; Fax: +46 46 17 82 51; E-mail: david.grubb@med.lu.se
Citation: Grubb D, Frigyesi A, Finnhult M, Dencker D, Olsson SG, Lindberg L. Breath Alcohol Analysis by Standardization to Water Vapour Enables Contact Free Sampling with Preserved High Accuracy and Precision As Compared With Mouthpiece Sampling. J Forensic Investigation. 2014;2(1): 6.
Copyright © 2014 Grubb D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Forensic Investigation | ISSN: 2330-0396 | Volume: 2, Issue: 1
Submission: 31 January, 2014| Accepted: 17 April, 2014 | Published: 19 April, 2014
Abstract
A novel breath analyzer has been developed that determines the breath alcohol concentration (BrAC) by tandardizing the exhaled alcohol and water vapour concentrations to the fully saturated water vapour concentration at 37 ºC (44 mg/L). The method enables the determination of a person’s BrAC without the use of a mouthpiece, i.e. contact free sampling. This, however, requires that the amount of breath sample dilution with ambient air is correctly determined. The main purpose of the study was to evaluate the impact of ambient air dilution on the analytical performance of the analyzer.Instrument performance was evaluated at vapour-alcohol concentrations ranging from 0.030 to 2.000 mg/L using a prototype simulator mimicking natural exhalations. The simulator outlet was either connected to the inlet of the analyzer (mouthpiece simulation) or placed at a distance of 50 and 100 mm (contact free simulation).
The accuracy was > 98 % and the precision (coefficient of variation, CV) was < 0.3 % in the mouthpiece simulations at concentrations above 0.1 mg/L. In the contact free simulations from 50 and 100 mm the corresponding accuracy was > 98 and 97 % and the CVs were < 0.7 and 1 %, respectively (n = 90).
A human drinking study was also performed where seven subjects drank 0.5 g alcohol/kg bodyweight, then providing 168 mouth piece and contact free samples from a distance of 50 and 100 mm. There was no difference between the BrACs of samples from 50 mm and the mouthpiece samples (mean 0.239 mg/L). The BrACs of samples from 100 mm were negligibly lower (0.234 mg/L).
Breath alcohol analysis by standardization to water vapour enables contact free sampling with preserved high accuracy and precision as compared with mouthpiece sampling.
The analyzer consists of a rectangular metallic box enclosing a cylindrical measuring chamber (Figure 1). At the back of the analyzer a fan system continuously sucks ambient air into the analyzer therebyflooding the measuring chamber with air. During exhalation, the fan system is closed by a check valve and the breath sample passes through the measuring chamber. As the exhalation ceases, the valve reopens and the analyzer is again flushed with ambient air.
Infrared light is transmitted through the measuring chamber. In front of the light detector a rotating disc is placed on which six filters are mounted. Three filters allow the passage of wavelengths of 3.32, 3.40 and 3.49 μm to allow for discrimination and calculation of alcohol by means of infrared absorption. Three additional filters are mounted on the disc, one reference filter (3.70 μm), one for determination of water vapour (2.58 μm) and one for determination of CO2 (4.40 μm). The disc spins at a rate of 33 Hz. The concentrations of alcohol, water vapour and CO2 are thus determined 33 times per second.
The breath sample is recognised by means of an increase in the CO2 concentration to a preset minimum level which will guarantee that the sample contains deep lung air. This triggers a measurement that includes all the data points obtained from three seconds before the triggering-on time-point until three seconds after the CO2 concentration has fallen below a triggering-off level.
The computer software now plots the alcohol concentrations as a function of the simultaneously measured water vapour concentrations. This scatter plot will extend from the position of the ambient alcohol (zero) and water vapour concentrations to the concentrations of water vapour and alcohol in the breath sample. A “best straight line” is then fitted to the data points in the scatter plot by least squares linear regression. The straight line is extrapolated to the position of a water vapour concentration of 44 mg/L and the corresponding alcohol concentration is read off and reported as the BrAC of the breath sample (Figure 2).
The analyzer stores all the data from each measurement on a hard disc. The measurement can directly and retrospectively be examined to confirm its accuracy.
The subject can either exhale directly into the inlet of the analyser or through a mouthpiece which can be connected to the inlet. The mouthpiece consists of a plastic tube with a length of 60 mm, an outer diameter of 18 mm and an inner diameter of 16 mm.
Lung simulator
Since commercial wet bath simulators cannot guarantee a specific water vapour concentration [6,7], a prototype simulator was constructed (Figure 3). It consists of a cylinder made of stainless steel with an inner diameter of 100 mm and a length of 600 mm. Each end is closed by an aluminium block. The outside of the cylinder body and the end blocks are heated by elements to maintain an inner temperature of 46ºC. The heating elements are regulated by a microprocessor with input from four thermistors monitoring the simulator temperature at different sites. The body of the cylinder is wrapped with insulating material. The front end of the cylinder is perforated by an outlet pipe, a vaporizer, a carrier gas inlet and a differential pressure transducer. The back end is perforated by the shaft of the piston. On the length of the shaft there is a scale marking every ten mL, denoting the volume that is enclosed in the simulator. The piston slides almost without friction against the interior of the cylinder, allowing full expansion of gas being heated to 46ºC and thus the equilibration of the gas pressure to the ambient pressure. Completeness of pressure equilibration is monitored by the pressure transducer. The carrier gas hose is connected to the simulator via a stopcock valve on the outside. The fluid to be vaporized is put in an aluminium ampoule. The ampoule is turned upside down and placed on the seat of the vaporizer which is heated to 145ºC by a thermostat circuit. The ampoule is tightly fixed to the vaporizer by a screw pushing the rear end of the ampoule. The vapour is drained from the vaporizer into the simulator chamber via an orifice with a diameter of 0.3 mm. The vaporizer is thermally isolated from the simulator to avoid overheating. To empty the simulator the stopcock inside the outlet pipe is opened and the piston shaft is manually pushed into the cylinder. The outer diameter of the outlet pipe is 18 mm, the same as that of the mouthpiece.
Alcohol and water vapour calibration
The analyzer was first calibrated with ten different concentrations of water vapour in the concentration range of 5-49 mg/L. The carrier gas during calibration consisted of desiccated ambient air. The analyzer was then calibrated by vaporized weight/weight solutions of alcohol containing 0.050; 0.100; 0.200; 0.800 and 1.600 g alcoholper 44 g water (Elpako AB, Sweden). The absolute water vapour concentration can be determined directly by the analyzer after the water vapour calibration. Since the concentration ratio between alcohol and water vapour in the gas mixture is known, the infrared absorption corresponding to a certain vapour-alcohol concentration can be calculated by the computer software.
Simulations
The test gases were prepared by first pre-filling the lung simulator to a certain start volume with breath gas as the carrier gas. The breath gas contained 4 % CO2; 15.9 % O2; 0.7 % Argon and 79.4 % N2 (Air Liquide Gas AB, Sweden). The concentrations of the solutions used were 0.030; 0.120; 0.484; 0.978 and 2.000 g alcohol per 44 g water (Elpako AB, Sweden). 0.088 mL of the alcohol/water solution was then vaporised into the pre-filled simulator which took about one minute. The gas volume obtained from vaporizing 0.088 mL of each of the solutions were given by the ideal gas law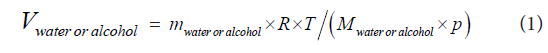
where Vwater or alcohol is the gas volume of water or alcohol (mL), mwater or alcohol is the mass of water or alcohol (g), R is the universal gas constant (J/molK), T is the temperature (Kelvin), Mwater or alcohol is the molar mass of water or alcohol (g/mol) and p is the atmospheric pressure (kPa). The total gas volume that yielded a water vapour concentration of 44 mg/L at 37ºC varied between 1942 and 2052 mL depending on what alcohol solution was used.
After the alcohol solutions had vaporised and the gas in the simulator had equilibrated to the ambient pressure, the gas volume was emptied into the analyzer in three approximately equal volumes. The partial volumes were insufflated into the analyzer either with the outlet pipe of the simulator connected to the analyzer inlet or with the outlet placed at a distance of 50 and 100 mm from the analyzer inlet. The sequence of the three different simulations was randomized to avoid possible bias. The insufflations were made in a time frame corresponding to only fractions of a second. The outlet of the test lung was opened and closed immediately before and after the insufflations to avoid ambient air leakage into the simulator. Each alcohol solution was vaporised 30 times, yielding altogether 90 vapour-alcohol determinations. Pure water was vaporised 12 times, yielding 36 determinations.
Exhalations
Seven volunteers, one female and six males, with ages ranging from 19 to 75 years and body weights ranging from 52 to 87 kg gave their informed consent. The subjects were fasted at least for two hours following a light meal. The subjects drank 0.5 g alcohol per kg bodyweight within 15 minutes. The alcohol drink was made from gin (40 % v/v) which was diluted by an equal volume of a non-alcohol containing soft drink. Ten minutes after finishing the alcohol drink, the subjects started to provide breath samples into the analyzer every 10 minutes through the mouthpiece. 60 minutes after the alcohol drink the BrACs of all the subjects were decreasing, indicating that the distribution or elimination phase of blood alcohol was reached. The subjects were now instructed to exhale either through the mouthpiece or directly into the inlet of the analyzer from a distance of 50 or 100 mm after the mouthpiece had been removed. The distance between the exhaling subject and the analyzer inlet was set by positioning a 50 or 100 mm long plastic straw between the chin of the subject and the analyzer front beneath the inlet.
These three different exhalations comprised one series of exhalations. The exhalations within one series were separated by 30 seconds. The third and the first exhalation of two following series were separated by two minutes. Each subject furnished 24 series of exhalation. A total number of 168 contact free exhalations from a distance of 50 and 100 mm and 168 mouthpiece exhalations were thus obtained. The order of the different exhalations within the series was randomized to avoid bias due to procedure. The mouthpiece was kept in a warming box heated to 60ºC in between the measurements.
Dilution
To confirm that the contact free simulations and exhalations caused a significant amount of ambient air dilution, the recorded maximum alcohol concentration during each individual simulation and exhalation were identified. The maximum concentrations were pooled for each group and presented as a mean value.
Data analysis
The precision of the vapour-alcohol determinations was measured with the coefficient of variation (CV)
Where SD is the standard deviation and C is the mean concentration.
Since the variables were normally distributed according to the Kolmogorov-Smirnov test, the paired t-test was used to test for differences between the vapour-alcohol readings, the BrACs and the maximum alcohol concentrations. The F-test was used to test for group-wise differences in CV-values. The Bonferroni method was used to decrease the probability of falsely significant results due to multiple comparisons. P-values < 0.05 were taken to indicate statistical significance.
The differences between BrACs obtained by mouthpiece and contact free exhalations were visualised in Bland-Altman plots. The 95 % limits of agreement (LOA) were calculated as 1.96 * the standard deviation of the differences.
The target vapour-alcohol concentrations were underestimated by 0-3.3 % in the mouthpiece simulations (Table 1). The contact free simulations from 50 mm read the vapour-alcohol concentrations 0.001-0.004 mg/L lower than the mouthpiece simulations. Furthermore, the simulations from a distance of 100 mm read the vapour-alcohol concentrations 0.001-0.010 mg/L lower than the simulations from 50 mm (Table 1) .
The precision (CV%) of the contact free simulations from 50 mm were slightly lower than the precision of the mouthpiece simulations although this did not reach statistical significance for most comparisons. The precision of simulations from a distance of 100 mm also tended to be slightly lower than the precision of simulations from 50 mm (Table 1).
Exhalations
The BrACs of the mouthpiece directed exhalations were not statistically different from the BrACs of contact free exhalations from a distance of 50 mm (mean BrAC = 0.239 mg/L for both groups) whereas the BrACs of contact free exhalations from 100 mm were significantly lower with respect to the third decimal digit (mean BrAC= 0,234; p < 0.001).
The Bland-Altman plots showed that the mean BrAC-differences were -0.0005 and 0.005 mg/L and the 95 % LOA were -0.018 to 0.017 and -0.009 to 0.020 mg/L between the mouthpiece and the contact free exhalations from a distance of 50 and 100 mm, respectively (Figure 4).
Dilution
A significant amount of ambient air dilution was present during the contact free simulations and exhalations as evidenced by the lower recorded maximum alcohol concentrations in the contact free groups. The amount of dilution was also significantly larger from a distance of 100 mm compared with 50 mm (Table 2).
Alcohol concentrations represent the mean of the recorded maximal concentrations from each individual simulation (n = 150) and exhalation (n = 168). The simulations with an alcohol concentration of 0 mg/L were omitted. Differences between groups were tested by paired t-test. a) Mouthpiece vs. contact free (50 mm) simulation. b)Contact free (50 mm) vs. contact free (100 mm) simulation. c) Contact free (100 mm) vs. mouthpiece simulation. d) Mouthpiece vs. contact free (50 mm) exhalation. e) Contact free (50 mm) vs. contact free (100 mm) exhalation. f) Contact free (100 mm) vs. mouthpiece exhalation. *** p < 0.001.
Keywords
Analytical performance; Vapour alcohol; Breath alcohol analysis; Standardization; Mouthpiece; Contact free samplingIntroduction
In standard forensic practice, quantitative breath alcohol analysis consists of the determination of the absolute, end-expiratory breath alcohol concentration (BrAC) as the test subject provides a prolonged exhalation into the analyzer through a mouthpiece [1]. A novel breath analyzer has been developed that standardizes the exhaled alcohol and water vapour to the deep lung water vapour concentration. This method relies on the fact that the water vapour in deep lung always is saturated to 100 % and the vapour concentration therefore is constant at 37 ºC (44 mg/L) [2,3]. The main benefit of breath alcohol analysis by standardization to water vapour is that the method enables contact free breath alcohol analysis, thereby obviating the need for a mouthpiece. As contact free analysis most likely would simplify the testing process and reduce associated costs, this method of analysis might prove to be a valuable analytical resource.Obviously, contact free exhalations cause a random dilution of the breath sample with ambient air. Consequently, the technique needs to determine the amount of dilution of the exhaled alcohol to be able to determine the “undiluted” BrAC. This is done by simultaneously measuring the exhaled alcohol and water vapour concentrations and constructing a concentration ratio between the gases throughout the exhalation. The “undiluted” BrAC can subsequently be determined by extrapolating the alcohol/water-ratio to the water vapour concentration of 44 mg/L. Ideally, this method perfectly compensates for any random dilution of the breath sample and the amount of dilution therefore has no impact on the resulting BrAC.
Contact free breath analysis based on standardization to water vapour has previously been validated in humans and shown to possess an excellent agreement with the coexisting arterial blood alcohol concentration and to be as precise as blood alcohol analysis [4,5]. However, the possible impact of the amount of ambient air dilution on the resulting BrAC has not been systematically evaluated.
The main purpose of the present study was to evaluate the impact of different amounts of ambient air dilution on the analytical performance of the analyzer. This was done by comparing the results of breath simulations where the outlet of the simulator was connected to the inlet of the analyzer with results obtained when the simulator was placed at a distance of 50 and 100 mm from the analyzer. A prototype simulator was utilized entailing a good resemblance with natural, unobstructed exhalations.
A human drinking study was also conducted to compare the BrACs of mouthpiece directed exhalations with BrACs obtained by contact free breath sampling at a distance of 50 and 100 mm from the analyzer.
Material and Methods
Breath analyzerThe analyzer consists of a rectangular metallic box enclosing a cylindrical measuring chamber (Figure 1). At the back of the analyzer a fan system continuously sucks ambient air into the analyzer therebyflooding the measuring chamber with air. During exhalation, the fan system is closed by a check valve and the breath sample passes through the measuring chamber. As the exhalation ceases, the valve reopens and the analyzer is again flushed with ambient air.
Infrared light is transmitted through the measuring chamber. In front of the light detector a rotating disc is placed on which six filters are mounted. Three filters allow the passage of wavelengths of 3.32, 3.40 and 3.49 μm to allow for discrimination and calculation of alcohol by means of infrared absorption. Three additional filters are mounted on the disc, one reference filter (3.70 μm), one for determination of water vapour (2.58 μm) and one for determination of CO2 (4.40 μm). The disc spins at a rate of 33 Hz. The concentrations of alcohol, water vapour and CO2 are thus determined 33 times per second.
The breath sample is recognised by means of an increase in the CO2 concentration to a preset minimum level which will guarantee that the sample contains deep lung air. This triggers a measurement that includes all the data points obtained from three seconds before the triggering-on time-point until three seconds after the CO2 concentration has fallen below a triggering-off level.
The computer software now plots the alcohol concentrations as a function of the simultaneously measured water vapour concentrations. This scatter plot will extend from the position of the ambient alcohol (zero) and water vapour concentrations to the concentrations of water vapour and alcohol in the breath sample. A “best straight line” is then fitted to the data points in the scatter plot by least squares linear regression. The straight line is extrapolated to the position of a water vapour concentration of 44 mg/L and the corresponding alcohol concentration is read off and reported as the BrAC of the breath sample (Figure 2).
Figure 2: Excerpts from the analyzer software illustrating the determination of an alcohol concentration of a contact free exhalation (a) and simulation (b) from a distance of 100 mm. Note the non-linear increase in alcohol concentration (1) due to different dead space for alcohol and water vapour, until deep lung air is exhaled, the so called “alveolar plateau” (circle). The breath sample is then washed out of the analyzer (2). The regression line (3) is extrapolated to a water vapour concentration of 44 mg/ml (4) which corresponds to the BrAC of the breath sample and the vapour-alcohol concentration of the simulator sample. In the simulations it is not possible to differentiate the increase in alcohol concentrations from the “wash out” phase. No “alveolar plateau” is present in the simulations.
The analyzer stores all the data from each measurement on a hard disc. The measurement can directly and retrospectively be examined to confirm its accuracy.
The subject can either exhale directly into the inlet of the analyser or through a mouthpiece which can be connected to the inlet. The mouthpiece consists of a plastic tube with a length of 60 mm, an outer diameter of 18 mm and an inner diameter of 16 mm.
Lung simulator
Since commercial wet bath simulators cannot guarantee a specific water vapour concentration [6,7], a prototype simulator was constructed (Figure 3). It consists of a cylinder made of stainless steel with an inner diameter of 100 mm and a length of 600 mm. Each end is closed by an aluminium block. The outside of the cylinder body and the end blocks are heated by elements to maintain an inner temperature of 46ºC. The heating elements are regulated by a microprocessor with input from four thermistors monitoring the simulator temperature at different sites. The body of the cylinder is wrapped with insulating material. The front end of the cylinder is perforated by an outlet pipe, a vaporizer, a carrier gas inlet and a differential pressure transducer. The back end is perforated by the shaft of the piston. On the length of the shaft there is a scale marking every ten mL, denoting the volume that is enclosed in the simulator. The piston slides almost without friction against the interior of the cylinder, allowing full expansion of gas being heated to 46ºC and thus the equilibration of the gas pressure to the ambient pressure. Completeness of pressure equilibration is monitored by the pressure transducer. The carrier gas hose is connected to the simulator via a stopcock valve on the outside. The fluid to be vaporized is put in an aluminium ampoule. The ampoule is turned upside down and placed on the seat of the vaporizer which is heated to 145ºC by a thermostat circuit. The ampoule is tightly fixed to the vaporizer by a screw pushing the rear end of the ampoule. The vapour is drained from the vaporizer into the simulator chamber via an orifice with a diameter of 0.3 mm. The vaporizer is thermally isolated from the simulator to avoid overheating. To empty the simulator the stopcock inside the outlet pipe is opened and the piston shaft is manually pushed into the cylinder. The outer diameter of the outlet pipe is 18 mm, the same as that of the mouthpiece.
Alcohol and water vapour calibration
The analyzer was first calibrated with ten different concentrations of water vapour in the concentration range of 5-49 mg/L. The carrier gas during calibration consisted of desiccated ambient air. The analyzer was then calibrated by vaporized weight/weight solutions of alcohol containing 0.050; 0.100; 0.200; 0.800 and 1.600 g alcoholper 44 g water (Elpako AB, Sweden). The absolute water vapour concentration can be determined directly by the analyzer after the water vapour calibration. Since the concentration ratio between alcohol and water vapour in the gas mixture is known, the infrared absorption corresponding to a certain vapour-alcohol concentration can be calculated by the computer software.
Simulations
The test gases were prepared by first pre-filling the lung simulator to a certain start volume with breath gas as the carrier gas. The breath gas contained 4 % CO2; 15.9 % O2; 0.7 % Argon and 79.4 % N2 (Air Liquide Gas AB, Sweden). The concentrations of the solutions used were 0.030; 0.120; 0.484; 0.978 and 2.000 g alcohol per 44 g water (Elpako AB, Sweden). 0.088 mL of the alcohol/water solution was then vaporised into the pre-filled simulator which took about one minute. The gas volume obtained from vaporizing 0.088 mL of each of the solutions were given by the ideal gas law
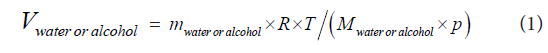
where Vwater or alcohol is the gas volume of water or alcohol (mL), mwater or alcohol is the mass of water or alcohol (g), R is the universal gas constant (J/molK), T is the temperature (Kelvin), Mwater or alcohol is the molar mass of water or alcohol (g/mol) and p is the atmospheric pressure (kPa). The total gas volume that yielded a water vapour concentration of 44 mg/L at 37ºC varied between 1942 and 2052 mL depending on what alcohol solution was used.
After the alcohol solutions had vaporised and the gas in the simulator had equilibrated to the ambient pressure, the gas volume was emptied into the analyzer in three approximately equal volumes. The partial volumes were insufflated into the analyzer either with the outlet pipe of the simulator connected to the analyzer inlet or with the outlet placed at a distance of 50 and 100 mm from the analyzer inlet. The sequence of the three different simulations was randomized to avoid possible bias. The insufflations were made in a time frame corresponding to only fractions of a second. The outlet of the test lung was opened and closed immediately before and after the insufflations to avoid ambient air leakage into the simulator. Each alcohol solution was vaporised 30 times, yielding altogether 90 vapour-alcohol determinations. Pure water was vaporised 12 times, yielding 36 determinations.
Exhalations
Seven volunteers, one female and six males, with ages ranging from 19 to 75 years and body weights ranging from 52 to 87 kg gave their informed consent. The subjects were fasted at least for two hours following a light meal. The subjects drank 0.5 g alcohol per kg bodyweight within 15 minutes. The alcohol drink was made from gin (40 % v/v) which was diluted by an equal volume of a non-alcohol containing soft drink. Ten minutes after finishing the alcohol drink, the subjects started to provide breath samples into the analyzer every 10 minutes through the mouthpiece. 60 minutes after the alcohol drink the BrACs of all the subjects were decreasing, indicating that the distribution or elimination phase of blood alcohol was reached. The subjects were now instructed to exhale either through the mouthpiece or directly into the inlet of the analyzer from a distance of 50 or 100 mm after the mouthpiece had been removed. The distance between the exhaling subject and the analyzer inlet was set by positioning a 50 or 100 mm long plastic straw between the chin of the subject and the analyzer front beneath the inlet.
These three different exhalations comprised one series of exhalations. The exhalations within one series were separated by 30 seconds. The third and the first exhalation of two following series were separated by two minutes. Each subject furnished 24 series of exhalation. A total number of 168 contact free exhalations from a distance of 50 and 100 mm and 168 mouthpiece exhalations were thus obtained. The order of the different exhalations within the series was randomized to avoid bias due to procedure. The mouthpiece was kept in a warming box heated to 60ºC in between the measurements.
Dilution
To confirm that the contact free simulations and exhalations caused a significant amount of ambient air dilution, the recorded maximum alcohol concentration during each individual simulation and exhalation were identified. The maximum concentrations were pooled for each group and presented as a mean value.
Data analysis
The precision of the vapour-alcohol determinations was measured with the coefficient of variation (CV)

Where SD is the standard deviation and C is the mean concentration.
Since the variables were normally distributed according to the Kolmogorov-Smirnov test, the paired t-test was used to test for differences between the vapour-alcohol readings, the BrACs and the maximum alcohol concentrations. The F-test was used to test for group-wise differences in CV-values. The Bonferroni method was used to decrease the probability of falsely significant results due to multiple comparisons. P-values < 0.05 were taken to indicate statistical significance.
The differences between BrACs obtained by mouthpiece and contact free exhalations were visualised in Bland-Altman plots. The 95 % limits of agreement (LOA) were calculated as 1.96 * the standard deviation of the differences.
Results
SimulationsThe target vapour-alcohol concentrations were underestimated by 0-3.3 % in the mouthpiece simulations (Table 1). The contact free simulations from 50 mm read the vapour-alcohol concentrations 0.001-0.004 mg/L lower than the mouthpiece simulations. Furthermore, the simulations from a distance of 100 mm read the vapour-alcohol concentrations 0.001-0.010 mg/L lower than the simulations from 50 mm (Table 1) .
The precision (CV%) of the contact free simulations from 50 mm were slightly lower than the precision of the mouthpiece simulations although this did not reach statistical significance for most comparisons. The precision of simulations from a distance of 100 mm also tended to be slightly lower than the precision of simulations from 50 mm (Table 1).
Exhalations
The BrACs of the mouthpiece directed exhalations were not statistically different from the BrACs of contact free exhalations from a distance of 50 mm (mean BrAC = 0.239 mg/L for both groups) whereas the BrACs of contact free exhalations from 100 mm were significantly lower with respect to the third decimal digit (mean BrAC= 0,234; p < 0.001).
The Bland-Altman plots showed that the mean BrAC-differences were -0.0005 and 0.005 mg/L and the 95 % LOA were -0.018 to 0.017 and -0.009 to 0.020 mg/L between the mouthpiece and the contact free exhalations from a distance of 50 and 100 mm, respectively (Figure 4).
Figure 4: Difference of BrAC between mouthpiece (mp) and contact free exhalations from 50 (a) and 100 (b) mm plotted against the mean BrAC. Unbroken lines denote the mean difference. Hatched lines denote the 95 % limits of agreement (%LOA). 168 BrACs were determined by mouthpiece and contact free exhalations. Seven subjects drank 0.5 g alcohol per kg bodyweight.
Dilution
A significant amount of ambient air dilution was present during the contact free simulations and exhalations as evidenced by the lower recorded maximum alcohol concentrations in the contact free groups. The amount of dilution was also significantly larger from a distance of 100 mm compared with 50 mm (Table 2).
Alcohol concentrations represent the mean of the recorded maximal concentrations from each individual simulation (n = 150) and exhalation (n = 168). The simulations with an alcohol concentration of 0 mg/L were omitted. Differences between groups were tested by paired t-test. a) Mouthpiece vs. contact free (50 mm) simulation. b)Contact free (50 mm) vs. contact free (100 mm) simulation. c) Contact free (100 mm) vs. mouthpiece simulation. d) Mouthpiece vs. contact free (50 mm) exhalation. e) Contact free (50 mm) vs. contact free (100 mm) exhalation. f) Contact free (100 mm) vs. mouthpiece exhalation. *** p < 0.001.
Discussion
The main finding of the present study was that alcohol analysis by standardization to water vapour compensated excellently for the random dilution with ambient air caused by contact free sampling. It is acknowledged, though, that the contact free simulations caused the analyzer to read slightly lower alcohol concentrations compared with the mouthpiece simulations. The absolute concentration differences, however, were only 0.001-0.004 mg/L between the mouthpiece and the contact free simulations from a distance of 50 mm. Furthermore, there was a tendency that increasing amounts of dilution caused the analyzer to read lower alcohol concentrations, as evidenced by the concentration differences between simulations from a distance of 50 and 100 mm. The explanation to why standardization to water vapour did not perfectly compensate for the sample dilution may be that the dilution of alcohol vapour was not identical to that of water vapour. This may be caused by the fact that ambient air contains a certain amount of humidity but no alcohol which creates a steeper relative concentration gradient for alcohol to ambient air than for water vapour.Generally, the target vapour-alcohol concentrations were underestimated in the mouthpiece simulations. This may be explained by the fact that the analyzer was calibrated with desiccated air whereas it was tested with breath gas containing CO2 which may have caused asmall interference on the measurements of water vapour or alcohol.
In contrast to the simulations, the human drinking study did not show any significant differences between the BrACs of mouthpiece and contact free exhalations from a distance of 50 mm. There was, however, a difference with respect to the third decimal digit compared with the contact free exhalations from a distance of 100 mm. Although statistically significant, these small differences are of no practical forensic relevance since the third decimal digit in practice is truncated and not reported [8,9].
To be able to calibrate and test the analyzer, a prototype simulator with the ability of providing gas mixtures containing both alcohol and water vapour at specified concentrations had to be constructed. In addition, the shift emptying of the gas mixture inside the simulator mimicked natural unobstructed exhalations. The test procedure therefore rigorously validates that the analyzer is capable of correctly measuring a vapour sample that is delivered under a short time frame and with a non-constant flow rate. In contrast, most commercial simulators work by flooding the analyzer with a constant and prolonged flow of alcohol vapour [6 7]. Consequently, such a procedure cannot validate that the tested analyzer is capable of correctly measuring the BrAC of a brief natural exhalation.
However, the use of a prototype simulator is also a limitation of the study since the analyzer neither can be directly compared with other analyzers evaluated with commercial simulators [10-12] nor validated against the various standards that stipulate the analytical performance required for evidential breath testing [9,13,14]. These standards are valid only for the determination of the absolute BrAC determined at the end-expiratory portion of a prolonged exhalation through a mouthpiece
.Nevertheless, it is interesting to note that for evidential purposes, the OIML R 126 [9] stipulates for concentrations between 0-2.00 mg/L the maximum permissible error to be 0.020 mg/L or 5 % of the reference value, whichever is the greater. In the present study the systematic errors of simulations from 100 mm were 0.003 mg/L at a concentration of 0.030 mg/L and 2.3 % at a concentration of 2.000 mg/L.
Besides water vapour, CO2 has been suggested as a tracer gas to enable contact free breath alcohol analysis [15]. Unfortunately, there is a large variability of the expired CO2 concentration between healthy individuals which is why the use of CO2 as a means to estimate alveolar air was abandoned already 1967 [16]. In addition, exhaled CO2 varies more with the pre-test breathing pattern than alcohol does [17]. The standardization of alcohol to a predefined CO2 concentration therefore introduces methodological flaws. As water vapour is reliably fully saturated at core body temperature [2,3] and core body temperature is a strictly regulated parameter, the water vapour concentration of 44 mg/L provides a more stable reference value than a predefined CO2 concentration. However, this does not mean that standardizing alcohol to the alveolar water vapour concentration determines the true alveolar alcohol concentration. The reason for this is that proportionally more alcohol than water vapour is resorbed back to the airway mucosa during exhalation [18-20]. As is the case with the current practice of determining an absolute exhaled alcohol concentration, standardizing alcohol to water vapour also underestimates the true alveolar alcohol concentration, albeit to a slightly lesser degree.
In conclusion, it is demonstrated here that breath alcohol analysis by standardization to deep lung water vapour enables contact free determination of the BrAC with an equally good analytical performance as mouthpiece directed analysis. This novel technique of breath alcohol analysis is now under field evaluation at border controls of ferry terminals in Sweden.
Acknowledgements
This study was made possible in part by grants from “Anna och Edwin Bergers stiftelse”.References
- Hlastala M P (1998) The alcohol breath test - a review. J Appl Physiol 84: 401-408.
- Williams R, Rankin N, Smith T, Galler D, Seakins P (1996) Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med 24: 1920-1929.
- McFadden E R (1983) Respiratory heat and water exchange: physiological and clinical applications. J Appl Physiol 54: 331-336.
- Lindberg L, Brauer S, Wollmer P, Goldberg L, Jones AW, et al. (2007) Breath alcohol concentration determined with a new analyzer using free exhalationpredicts almost precisely the arterial blood alcohol concentration. Forensic Sci Int 168: 200-207.
- Grubb D, Rasmussen B, Linnet K, Olsson SG, Lindberg L (2012) Breathalcohol analysis incorporating standardization to water vapour is as preciseas blood alcohol analysis. Forensic Sci Int 216: 88-91.
- Dubowski KM (1979) Breath-alcohol simulators: Scientific basis and actual performance. J Anal Toxicol 3: 177-182.
- Dubowski KM, Essary NA (1991) Evaluation of commercial breath-alcoholsimulators: further studies. J Anal Toxicol 15: 272-275.
- Dubowski K M (1994) Quality assurance in breath-alcohol analysis. J Anal Toxicol 18: 306-311.
- OIML R 126 International recommendation: Evidential breath analyzers(2012) Bucharest 1-69.
- Dubowski KM, Essary NA (1999) Measurement of low breath-alcoholconcentrations: Laboratory studies and field experience. J Anal Toxicol 23:386-395.
- Razatos G, Luthi R, Kerrigan S (2005) Evaluation of a portable evidential breath alcohol analyzer. Forensic Sci Int 153: 17-21.
- Frankvoort W, Mulder JA, Neuteboom W (1987) The laboratory testing of evidential breath-testing (EBT) machines. Forensic Sci Int 35: 27-43.
- NHTSA. Model specifications for devices to measure breath alcohol. Federal register (1993) 58: 48705-48710.
- Recommended standards and test procedures of the Canadian society of forensic science alcohol test committee. (2003) Can Soc Forensic Sci J 36:101-127.
- Jonsson A, Hok B, Andersson L, Hedenstierna G (2009) Methodologyinvestigation of expirograms for enabling contact free breath alcohol analysis.J Breath Res 3: 1-8.
- Mason MF, Dubowski KM (1976) Breath-alcohol analysis: uses, methods,and some forensic problems-review and opinion. J Forensic Sci 21: 9-41.
- Kaisdotter Andersson A, Hok B, Ekstrom M, Hedenstierna G (2011) Influence from breathing pattern on alcohol and tracer gas expirograms - implicationsfor alcolock use. Forensic Sci Int 206: 52-57.
- Bui T, Dabdub D, George SC (1998) Modeling bronchial circulation withapplication to soluble gas exchange: description and sensitivity analysis. J Appl Physiol 84: 2070-2088.
- Anderson JC, Babb AL, Hlastala MP (2003) Modeling soluble gas exchange in the airways and alveoli. Ann Biomed Eng 31: 1402-1422.
- Lindberg L, Grubb D (2012) Simultaneously recorded single-exhalation profiles of ethanol, water vapour and CO2 in humans: impact of pharmacokinetic phases on ethanol airway exchange. J Breath Res 6: 1-7.







