Journal of Surgery
Download PDF
Research Article
*Address for Correspondence: Jessica L. Buicko, Department of Surgery, Miller School of Medicine, University of Miami, 5301 S Congress Ave. Atlantis, FL 33462, USA, Tel: 5182297711; Fax: 5615481743; E-mail: jbuicko@med.miami.edu
Citation: Kichler K, Buicko JL, De La Cruz LM, Moody A, Kaza S. Robotic Versus Laparoscopic Sleeve Gastrectomy for Morbid Obesity: A Meta-Analysis. J Surgery. 2017;5(1): 5.
Copyright © 2016 Kichler K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 5, Issue: 1
Submission: 28 November, 2016 | Accepted: 02 January, 2017 | Published: 10 January, 2017
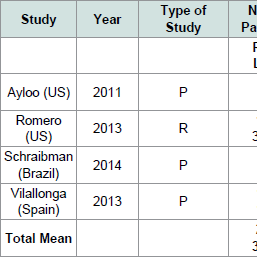
The characteristics of each selected study are summarized in ( Table 1). Three studies had a prospective design and one study had a retrospective design. Mean patient age for each study ranged from 38-46 years. Four studies comprised 3,599 sleeve gastrectomy cases. The sample size of studies ranged from 48 to 3,282 patients. In total, 280 cases were RSG and 3,319 were LSG. Mean preoperative BMI ranged from 39.4 to 56 kg/m2 and 41.3 to 57 kg/m2 for LSG and RSG, respectively.
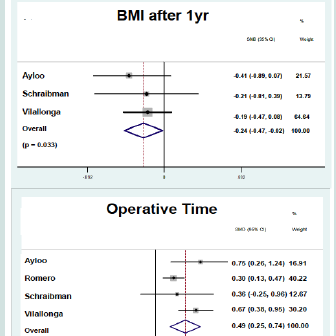
Outcomes of each study are documented in ( Table 2), and metaanalysis forest plots are demonstrated in ( Figure 2). Four studies reported operative time. Operative time was significantly greater for patients undergoing RSG (SMD: 0.494; 95% CI: 0.245-0.743; p < 0.01). Four studies reported length of stay. There was no statistically significant difference in length of stay for patient undergoing LSG and RSG (SMD: -0.062; 95% CI: -0.637-0.513; p = 0.833).Perioperative complications reported in studies included leakage from the staple line, bleeding and stricture formation. Two studies reported on leak rate and no statistically significant differences were found between patients undergoing LSG and RSG (RR: 0.43; CI: 0.11- 1.64; p = 0.218). Two studies reported on postoperative bleeding and no statistically significant differences were found between groups (RR: 0.58; CI: 0.16-2.07; p = 0.401). Two studies reported on stricture formation and no statistically significant differences were found between groups (RR: 1.81; CI: 0.25-13.13; p = 0.558).BMI after one year was reported in three studies. Weight loss was greater for patients undergoing RSG compared to LSG (SMD: -0.24; CI: -0.47- -0.02; p = 0.033).
Robotic Versus Laparoscopic Sleeve Gastrectomy for Morbid Obesity: A Meta-Analysis
Kandace Kichler1, Jessica L. Buicko1*, Lucy M. De La Cruz1, Alison Moody2 and Srinivas Kaza1
- 1Department of Surgery, University of Miami, Atlantis, FL, USA
- 2Miller School of Medicine, University of Miami, Atlantis, FL, USA
*Address for Correspondence: Jessica L. Buicko, Department of Surgery, Miller School of Medicine, University of Miami, 5301 S Congress Ave. Atlantis, FL 33462, USA, Tel: 5182297711; Fax: 5615481743; E-mail: jbuicko@med.miami.edu
Citation: Kichler K, Buicko JL, De La Cruz LM, Moody A, Kaza S. Robotic Versus Laparoscopic Sleeve Gastrectomy for Morbid Obesity: A Meta-Analysis. J Surgery. 2017;5(1): 5.
Copyright © 2016 Kichler K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Surgery | ISSN: 2332-4139 | Volume: 5, Issue: 1
Submission: 28 November, 2016 | Accepted: 02 January, 2017 | Published: 10 January, 2017
Abstract
Background: Sleeve gastrectomy (SG) represents the fastest growing bariatric surgical procedure and has been associated with few long term complications. SG provides the opportunity to act as a bridge for future procedures in the super obese, improving comorbidities before laparoscopic Roux-en-Y gastric bypass (RYGB). With the technological advancement in minimally invasive surgery, many surgeons have adopted the robotic technique. The purpose of this meta-analysis was to compare the clinical safety and efficacy of robotic sleeve gastrectomy (RSG) with laparoscopic sleeve gastrectomy (LSG).Methods: A MEDLINE database search was performed and selected studies included those in which RSG and LSG were compared in terms of perioperative outcomes. Evaluated variables included operative time, perioperative bleeding, length of stay, stricture formation, leak rate, and mean BMI after one year.
Results: Four studies matched the selection criteria and reported on a total of 3599 sleeve gastrectomy cases. Of these, 280 cases were RSG and 3319 were LSG. Comparing RSG to LSG, we found favorable outcomes in regards to mean BMI after one year (SMD: -0.243; 95% CI: -0.466-0.019; p = 0.033). However, operative time was increased (SMD: 0.602; 95% CI: 0.417-0.788; p < 0.01). Other results were not significant, including leak rate (RR: 0.433; 95% CI: 0.115-1.638; p = 0.218); perioperative bleeding (RR: 0.578; 95% CI: 0.161-2.075; p = 0.401); stricture formation (RR: 1.809; 95% CI: 0.249-13.132; p = 0.558); and length of stay (SMD: -0.078; 95% CI: -0.260-0.105; p = 0.404).
Conclusions: Robotic sleeve gastrectomy as compared to LSG shows a significantly increased operative time. In regards to mean BMI at one year, RSG is superior to LSG. There was no significant difference in LOS, perioperative bleeding, leak rate, or stricture formation. RSG is a safe and feasible alternative to conventional LSG [1-4].
Keywords
Bariatric surgery; Sleeve gastrectomy; Morbid obesity; Robotics; Meta-analysisLevel of Evidence
Level III- Meta-Analysis.Recommendation for Practice
RSG is a safe alternative to LSG. RSG is superior to LSG in regards to mean BMI at one year. With increased robotic experience, operative times for RSG should decrease.Introduction
Obesity rates have risen astronomically in the past decade. From 2009-2014, the prevalence of obese individuals increased by 2% so that in 2014, an astounding 27.7% of the population classified as obese. Obesity is one of the biggest drivers of chronic disease and as such, contributes significantly to both human morbidity and mortality and rising healthcare costs. Obesity is also the second leading cause of preventable death. If obesity rates continue to follow the current trajectory, by 2030, the cost of obesity-related comorbidities will be $66 billion annually [5].Bariatric surgery is the only proven modality to promote longterm weight loss in morbidly obese patients, and in doing so, also decreases the medical comorbidities that accompany obesity, namely diabetes mellitus, type 2. In light of this data, the National Institute of Health (NIH) issued a statement in 1991 stating that bariatric surgery was the most ideal treatment modality for patients with morbid obesity, defined as a body mass index (BMI) of 35 or higher [6,7].
Today, the sleeve gastrectomy (SG) is one of the surgical options offered to morbidly obese patients. The SG technique has evolved since the 1980s when Hess and Marceau et al. performed a preliminary SG as part of the larger Bilio-Pancreatric Diversion or Duodenal Switch (BPD-DS) procedure and noted significant weight loss in their patients. This discovery paved the way for the SG to be offered to patients undergoing Roux-en-Y Gastric Bypass (RYGB) as a “bridge” procedure. Then, in the early 1990s, with the advent of minimally invasive and laparoscopic surgery, the SG became a stand-alone surgical alternative for patients [8-13].
SG has many favorable attributes when compared to other bariatric surgical procedures and is reported to be associated with few long-term complications. Today, most patients undergoing SG are offered a laparoscopic procedure, which enhances visualization for the surgeon and provides the patient with a less painful postoperative course. The traditional open approach, on the other hand, is associated with a larger incision with difficult and often cumbersome closure and an increased length of stay. With the technological advancement of the da Vinci Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA), some surgeons have adopted the robotic technique, which further improves surgeon visualization, maneuverability, and better triangulation [14]. For these reasons, Robotic Sleeve Gastrectomy (RSG) is gaining popularity in the treatment of morbid obesity in Europe and North America. Nevertheless, some members of the surgical community criticize the RSG, stating that operating with the robot has a steep learning curve, which threatens to compromise patient outcomes [15]. The purpose of the current study is to perform a meta-analysis to compare the clinical safety and efficacy of RSG and LSG.
Methods
A search was conducted through the MEDLINE database using PubMed. Our search terms included: (“Gastrectomy/methods”[Mesh] OR “Gastrectomy/therapy”[Mesh])) AND (“Obesity”[Mesh] OR “Obesity, Abdominal”Mesh]). We filtered all articles from 1967 to 2015, selecting only those that contained the key terms “sleeve gastrectomy” and “obesity”. Additional searches were performed using the Google Scholar and Scopus databases. A manual forward and backward search of key relevant bibliographies was also performed. All searches were conducted in October 2014 and again in January 2015. Abstracts were screened to identify studies that included an internal comparison between RSG and LSG and reported on our outcomes of interest: operative time, perioperative bleeding, length of stay, stricture formation, leak rate, and/or mean BMI after one year.Data extraction
Two investigators performed the search and independently reviewed all selected articles and extracted data according to a prespecified protocol. Discrepancies in coding required a consensus between both investigators to be considered resolved.
Inclusion and exclusion criteria
Studies were selected based on the following inclusion criteria:
- Report on patients undergoing sleeve gastrectomy in the setting of morbid obesity [16]
- Include only adult patients (18 years and older)- Contain an internal comparison between RSG and LSGoutcomes
- Follow a prospective or retrospective cohort design
- Available in English
- Studies were excluded by any one of the following criteria:
- Non-human patients were included- Only non-surgical treatments were provided
- Meeting abstracts or Letters to the Editor
Statistical analysis
For all studies, we reported on one or more of the primary outcomes of interest (operative time, perioperative bleeding, length of stay, stricture formation, leak rate and mean BMI after one year). In addition, we extracted study type, year of publication, number of patients, mean patient age and mean preoperative BMI.
A study level meta-analysis was performed. Stata 12 software (StataCorp LP, College Station, TX) was used for all quantitative analyses. We used two different pooled statistics. Relative risk (RR) was determined for categorical outcomes, and standardized mean difference (SMD) was calculated for continuous outcomes.
For continuous variables, we reported the median and interquartile range. We then calculated the standardized mean difference (SMD) and the relative risk (RR) with the corresponding 95% confidence interval and p-value. The SMD is defined as the difference between the mean and standard deviation of the robotic group minus that of laparoscopic group for each study. The SMD was weighted by the sample size of each individual study.
For categorical variables, we first calculated the degree of heterogeneity across the studies and tested its significance using both Cochrane Q-test (significance level < 0.05) and the I squared statistic (> 50%) to determine the underlying statistical model. We calculated the relative risk (RR) of having an event for categorical outcomes using fixed effect model if no heterogeneity was present between studies or the random effects model when heterogeneity in study design was present. The effect size of each study was calculated by subtracting the proportion of affected individuals from those unaffected and dividing by the pooled standard deviation. A risk difference of zero favors the null hypothesis, meaning there is no difference in outcome measures between patients treated with either therapy. Statistically significant differences were considered at the p < 0.05 level.
Results
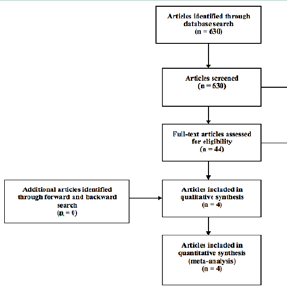
Description of studiesThe search yielded 630 publications of which 44 were fully reviewed and 4 met inclusion and exclusion criteria and were selected for our meta-analysis ( Figure 1).
The characteristics of each selected study are summarized in ( Table 1). Three studies had a prospective design and one study had a retrospective design. Mean patient age for each study ranged from 38-46 years. Four studies comprised 3,599 sleeve gastrectomy cases. The sample size of studies ranged from 48 to 3,282 patients. In total, 280 cases were RSG and 3,319 were LSG. Mean preoperative BMI ranged from 39.4 to 56 kg/m2 and 41.3 to 57 kg/m2 for LSG and RSG, respectively.
Outcomes of each study are documented in ( Table 2), and metaanalysis forest plots are demonstrated in ( Figure 2). Four studies reported operative time. Operative time was significantly greater for patients undergoing RSG (SMD: 0.494; 95% CI: 0.245-0.743; p < 0.01). Four studies reported length of stay. There was no statistically significant difference in length of stay for patient undergoing LSG and RSG (SMD: -0.062; 95% CI: -0.637-0.513; p = 0.833).
Ethical considerations
This study was performed according to the Helsinki declaration and approved by the Regional Ethical Review Board (registration number 2010/49). The study protocol was designed to meet the legislative documentation required by the country of origin.
The data were anonymized prior to calculations, and are presented in such a manner that it is impossible to identify or link to any specific individual. Therefore, it was not necessary to obtain approval from the individual patient’s guardians to conduct this study. It will not be possible to go back and trace or identify any of the participants.
Since this data was retrospectively collected from a prospectively collected database, the treatment plan of each patient was not altered. All evaluations, treatments, and procedures described in this report met the standard of care and were conducted at a tertiary center for pediatric surgery. No protocols were exercised that would have required appropriate informed consent or approval of an institutional review board. The risk of harming the patients in this study in a physical, social or psychological manner was nonexistent.
Discussion
In our meta-analysis we found no difference between RSG and LSG with regards to length of stay, stricture formation, perioperative bleeding, and leak rate. The mean BMI at 1-year was significantly less for patients undergoing RSG compared to LSG. Operative time was significantly longer in the RSG group.In terms of operative time, docking the robot is likely responsible for the time discrepancy between RSG and LSG groups. The docking time at each institution is both surgeon and staff dependent. A knowledgeable, trained staff can dock a robot in eight minutes, but due to inexperience, robot docking times have been reported as high as 20 minutes [14-16]. Console working time is also surgeon dependent and improves with surgeon experience. The learning curve for robotic surgery is less than that for laparoscopic surgery. It is possible that some of the surgeons performing RSG in our included studies were still improving their surgical efficiency and proficiency on the robot and this may have, in part, also accounted for the time difference between groups.
The difference in mean BMI after 1-year between groups was perplexing, as one would expect no difference in the mean BMI as a consequence of surgical approach. One hypothesis is that the visualization and triangulation provided by the robotic platform increases the precision of formation of the gastric sleeve. Perhaps, the laparoscopic approach has less maneuverability when retracting the stomach fundus from around the bougie to make a tight sleeve as compared to the robotic approach. As an alternative, our findings could represent a type I error. This result needs to be further investigated with larger studies to either confirm or refute the validity [17,18].
Our meta-analysis has limitations. First, all included studies had a retrospective or prospective cohort design and were not randomized controlled trials. Also, we performed a study-level, not individual-level meta-analysis, and as such, the study design may not be sufficiently sensitive to account for all biases. In addition, while all included studies contained an internal comparison between studies, only three studies included an internal comparison in which the same surgeons performed both the LSG and RSG procedures. In one study, RSGs performed at the author’s institution were compared to a systematic review of LSGs. This comparison arm is less valid, as differences in surgeon skill and technique, as well as differences in surgical institution are likely to play a role in outcome data.
An additional limitation is that portions of the surgical procedure varied between studies. For example, the method of reinforcing the staple line differed, with some surgeons over sewing the staple line, others utilizing a buttress material, and some surgeons chose to not reinforce the staple line. While staple line reinforcement has been shown to not affect complication rates in sleeve gastrectomy, ideally, surgical technique should be held constant between studies to make a more meaningful comparison [19]. Another important fact to note is that the robotic stapling portion of the procedures was similar to that in the laparoscopic - both arms in both studies utilized a laparoscopic linear bowel stapler to complete sleeve creation. This fact can argue that the comparison of leak rates is not valid, as the technique for staple line creation is effectively the same in both procedures.
Lastly, we utilized the outcomes of interest as defined by the individual study authors. Operative time was defined differently across studies with some authors including the robot docking time as a part of operative time, while other authors did not. For example, Vilallonga reported operative time and docking time as separate entities in their study. This meta-analysis would be stronger if outcome measures were uniformly defined across all included studies.
The use of robotic surgery for bariatric operations is still debated because of controversy surrounding true perioperative benefits and cost. In urologic and gynecologic surgery, the benefit of visualization and maneuverability in the pelvis help argue that robotic surgery is safer than the standard open or laparoscopic methods. General surgery has questionably limited value for robotic surgery with the exception of low colon resections and operations in the esophageal hiatus [20]. Although not demonstrated in the literature to date, we can reasonably hypothesize that the benefits associated with robotic operations in the esophageal hiatus can be extended to bariatric surgery as most of the critical dissection occurs in the hiatus near the angle of His. Triangulation in this area is difficult to achieve when using standard laparoscopic instruments. A more precise dissection in this area may allow for avoidance of surgical complications, such as postoperative bleeding and leaks. However, no differences were found between groups with regards to these complications in our meta-analysis.
Conclusion
Robotic sleeve gastrectomy for morbid obesity as compared to LSG shows a significantly increased operative time. In regards to mean BMI at one year, RSG is superior to LSG. There was no significant difference in terms of LOS, perioperative bleeding, leak rate, or stricture formation. RSG is a safe and feasible alternative to conventional LSG. Further comparative studies may shed additional light on perioperative outcomes.Strengths and Limitations
Robotic surgery has been shown to improve surgeon visualization, maneuverability, and triangulation but its use in bariatric surgery is controversial. This paper highlights the benefits of RSG in regards to mean BMI at one year compared to LSG. Strengths of our study include the high number of patients studied and the consistency in outcomes documented by the individual institutions, which allowed us to reasonably compare the two groups. There are no other studies of this nature in the current literature.This paper is a meta-analysis and there are intrinsic limitations in regards to heterogeneity of the studies. There are no published randomized control trials on the subject matter, which may impact the results. Nonetheless, the conclusions are relevant for future practice and further studies in regards to long-term outcomes of RSG in bariatric patients may shed additional light on perioperative outcomes.
Acknowledgements
We recognize the support of Leonardo Tamariz, MD, MPH from the University of Miami Department of Medicine in the development of this project. His assistance with the statistical analysis is greatly appreciated.References
- Ayloo S, Buchs NC, Addeo P, Bianco FM, Giulianotti PC (2011) Robot-assisted sleeve gastrectomy for super-morbidly obese patients. J Laparoendosc Adv Surg Tech A 21: 295-299.
- Romero RJ, Kosanovic R, Rabaza JR, Seetharamaiah R, Donkor C, et al. (2013) Robotic sleeve gastrectomy: experience of 134 cases and comparison with a systematic review of the laparoscopic approach. Obes Surg 23: 1743-1752.
- Schraibman V, Macedo AL, Epstein MG, Soares MY, Maccapani G, et al. (2014) Comparison of the morbidity, weight loss, and relative costs between robotic and laparoscopic sleeve gastrectomy for the treatment of obesity in Brazil. Obes Surg 24: 1420-1424.
- Vilallonga R, Fort JM, Caubet E, Gonzalez O, Armengol M (2013) Robotic sleeve gastrectomy versus laparoscopic sleeve gastrectomy: a comparative study with 200 patients. Obes Surg 23: 1501-1507.
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M (2011) Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378: 815-825.
- Buchwald H, Consensus Conference Panel (2005) Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis 1: 371-381.
- (1991) NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 115: 956-961.
- Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, et al. (1998)Biliopancreatic diversion with duodenal switch. World J Surg 22: 947-954.
- Ren CJ, Patterson E, Gagner M (2000) Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg 10: 514-523.
- De Csepel J, Burpee S, Jossart G, Andrei V, Murakami Y, et al. (2001) Laparoscopic biliopancreatic diversion with a duodenal switch for morbid obesity: a feasibility study in pigs. J Laparoendosc Adv Surg Tech A 11: 79-83.
- Aggarwal S, Kini SU, Herron DM (2007) Laparoscopic sleeve gastrectomy for morbid obesity: a review. Surg Obes Relat Dis 3: 189-194.
- Hess DS, Hess DW (1998) Biliopancreatic diversion with a duodenal switch. Obes Surg 8: 267-282.
- Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, et al. (2006) Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for highrisk patients with morbid obesity. Surg Endosc 20: 859-863.
- Cirocchi R, Boselli C, Santoro A, Guarino S, Covarelli P, et al. (2013) Current status of robotic bariatric surgery: a systematic review. BMC Surg 7: 13-53.
- Diamantis T, Alexandrou A, Nikiteas N, Giannopoulos A, Papalambros E (2011) Initial experience with robotic sleeve gastrectomy for morbid obesity. Obes Surg 21: 1172-1179.
- Gumbs AA, Gagner M, Dakin G, Pomp A (2007) Sleeve gastrectomy formorbid obesity. Obes Surg 17: 962-969.
- Hanly EJ, Talamini MA (2004 ) Robotic abdominal surgery. Am J Surg 188(4A Suppl): 19S-26S.
- Buchwald H, Oien DM (2009) Metabolic/bariatric surgery worldwide 2008. Obes Surg 19: 1605-1611.
- Knapps J, Ghanem M, Clements J, Merchant AM (2013) A systematic review of staple-line reinforcement in laparoscopic sleeve gastrectomy. JSLS 17: 390-399.
- Sgarbura O, Tomulescu V, Blajut C, Popescu I (2013) A 5-year perspective over robotic general surgery: indications, risk factors, and learning curves. Chirurgia (Bucur) 108: 599-610.