Journal of Toxins
Download PDF
Research Article
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 APC Road, Kolkata 700009, India, Tel: 91-33-23508386/(M) 09433139031; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Ghosh S, Saha K, Dasgupta SC, Gomes A. In vitro and In vivo Anti-Arthritic and Anti-Inflammatory Activity of Bungarus Fasciatus Venom. J Toxins. 2015;2(1): 5.
Copyright © 2015 Gomes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | Volume: 2, Issue: 1
Submission: 13 April 2015 | Accepted: 09 May 2015 | Published: 15 May 2015
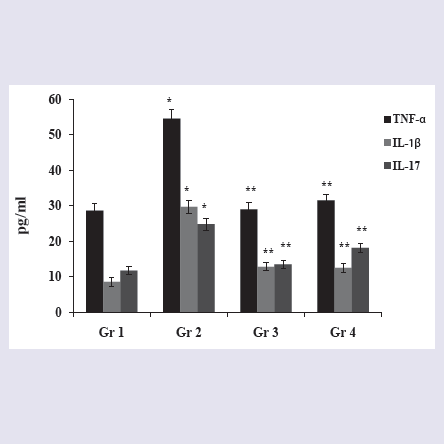
In vivo anti-arthritic activityEffect of BFV on serum proinflammatory cytokines: BFV significantly reduced serum pro inflammatory cytokines TNF-α, IL- 1β and IL-17 by 42.17%, 58.14% and 26.91% respectively; whereas standard drug, Indomethacin showed a reduction of TNF-α, IL-1β and IL-17 by 46.83%, 57.07% and 45.81% respectively when compared to arthritic control rats (Figure 2).
In vitro anti-inflammatory activity
In vivo anti-inflammatory activity
In vitro and In vivo Anti-Arthritic and Anti-Inflammatory Activity of Bungarus Fasciatus Venom
Susmita Ghosh1, Kalyani Saha1, Subir Chandra Dasgupta2, and Antony Gomes1*
- 1Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 A P C Road, Kolkata 700009, India
- 2Department of Zoology, Maulana Azad College, Kolkata, West Bengal, India
*Address for Correspondence: Antony Gomes, Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, 92 APC Road, Kolkata 700009, India, Tel: 91-33-23508386/(M) 09433139031; Fax: 91-33-2351-9755/2241-3288; E-mail: agomescu@gmail.com
Citation: Ghosh S, Saha K, Dasgupta SC, Gomes A. In vitro and In vivo Anti-Arthritic and Anti-Inflammatory Activity of Bungarus Fasciatus Venom. J Toxins. 2015;2(1): 5.
Copyright © 2015 Gomes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Toxins | Volume: 2, Issue: 1
Submission: 13 April 2015 | Accepted: 09 May 2015 | Published: 15 May 2015
Abstract
In the Indian traditional medicinal system Ayurveda and Unani, the use of snake venom has been mentioned to treat various diseases including arthritis. This prompted us to investigate the anti-arthritic andanti-inflammatory activity of banded krait venom (BFV). In the present study, in vitro anti-arthritic activity of BFV was evaluated by bovine serum protein denaturation and egg albumin denaturation and in vivo antiarthritic activity was evaluated by collagenase induced arthritis in male albino Wistar rats. In vitro anti-inflammatory activity was evaluated by RBC membrane stabilization and in vivo anti-inflammatory activity was evaluated by histamine/ formalin induced paw edema in male albino Swiss mice. BFV treatment significantly inhibited bovine serum protein and egg albumin denaturation. In collagenase induced arthritis model, BFV treatment significantly reduced serum proinflammatory cytokines TNF-α, IL-1β and IL-17, significantly increased percentage stabilization of RBC membrane and significantly decreased histamine/ formalin induced paw edema. The findings of the present study showed that Bungarus fasciatus venom possesses significant in vitro and in vivo antiarthritic and anti-inflammatory activities and may serve as a natural biomedicinal remedial agent against inflammatory conditions.Keywords
Bungarus fasciatus; Banded krait; Snake venom; Antiarthritis; Anti-inflammatoryIntroduction
Inflammation, a body defence mechanism is triggered by several conditions including injury. Macrophages and neutrophils take part in acute inflammation while T-cells and plasma cells are leading candidates in chronic inflammation [1]. Osteoarthritis, the most common type of arthritis [2], is most common in the knee joint (6%) and hip joint (3%) [3]. Clinical symptoms include joint pain, swelling and stiffness which may worsen after activity or inactivity [4]. Many studies have indicated that inflammation of the synovium may play an important role in the pathogenesis of OA [5,6]. Activated synoviocytes, macrophages and articular cartilage itself produce of a variety of proinflammatory cytokines like TNF-α and IL-1β contributing to tissue destruction [7,8]. Rheumatoid arthritis, another form of inflammatory arthritis, involves chronic inflammation of the synovial membrane in the joints of hands and feet with subsequent cartilage and bone erosion and destruction [9]. Nearly 1% of the world population is affected by rheumatoid arthritis [10]. Interplay between pro- and anti-inflammatory cytokines contribute to the pathogenesis in RA. There are many anti-inflammatory drugs (NSAIDs) and antiarthritic drugs (DMARDs) that have wide applications in clinical conditions [11] and are associated with several side effects like G.I tract complications, ulcers, cardiovascular problems [12,13]. Therefore, alternative therapies from natural resources are ventured throughout the world.Therapeutic use of snake venom to treat various diseases including arthritis has been mentioned in age old medicinal systems like Ayurveda, Unani and folk medicine [14]. Snake venom protein toxins like NKCT-1, NN-32 showed anti-arthritic activity in experimental animal models [15]. Bungarus fasciatus, one of the member of elapidae snake family, is distributed throughout eastern India including West Bengal. Though its venom contains many lethal cardiotoxic, neurotoxic compounds, several research works has been carried out with its venom which revealed its potential to treat various diseases. Bungarus fasciatus venom possesses an anticancer compound that could induce apoptosis of cancer cells and arrest cellcycle progression in the check points [16]. An antibiotic compound named Cathelicidin BF was isolated from Bungarus fasciatus venom [17]. But there is no scientific evidence of using Bungarus fasciatus venom to treat arthritis. The present research work was aimed to evaluate the in vitro and in vivo anti-arthritic and anti-inflammatory activity of Bungarus fasciatus venom in experimental models.
Materials and Methods
Chemicals and drugsDiclofenac sodium used under the brand name Voveran (Novartis, India). Other chemicals and reagents used are- BSA, Histamine, Collagenase (Sigma-aldrich, USA); Formaldehyde and Indomethacin (SRL, India); TNF-α, IL-1β and IL-17 ELISA kits (R & D Systems, USA). All other chemicals and reagents used were of analytical grade.
Collection and preparation of venom
Lyophilized Bungarus fasciatus venom (BFV) was commercially purchased from Calcutta snake park, Kolkata, India. A concentration of 1mg/ ml was achieved by dissolving lyophilized BFV in 0.9% NaCl.
Animals
Adult Wistar albino male rats (120±10 g) and Swiss albino male mice (20 ± 2 g) were purchased from approved animal breeders. They were kept in polypropylene cages, temperature maintained at 25±2 ºC, relative humidity 65% and given standard pellet diet and water ad libitum. Animal ethical clearance was availed before all experiments (IAEC-III/ Proposal/ AG-02/2012 dated 07.06.2012).
In vitro anti-arthritic activity
In vitro anti-arthritic activity of BFV was evaluated using bovine serum protein denaturation method [18] and egg albumin denaturation method [18].
Bovine serum albumin (BSA) denaturation method: Test solution (0.5 ml): 0.5% w/v aqueous solution of BSA (0.45 ml) and test solution (0.05 ml) of different concentrations were used.
Test control solution (0.5 ml): 0.5% w/v aqueous solution of BSA (0.45 ml) and distilled water (0.05 ml) were used.
Product control (0.5 ml): 0.45 ml distilled water and test solution (0.05 ml) of different concentrations were used.
Standard solution (0.5 ml): 0.5% w/v aqueous solution of BSA (0.45 ml) and diclofenac sodium (0.05 ml) of different concentrations were used.Test solution (0.05 ml) of different concentrations (2, 5, 10, 20, 30 and 60 μg/ml) and standard drug diclofenac sodium (0.05 ml) of different concentrations (2, 5, 10, 20, 30 and 60 μg/ml) were mixed with 0.5% w/v aqueous solution of BSA (0.45 ml). Then the samples were incubated at 37 ºC for 20 min followed by incubation at 57 ºC for 3 min. 2.5 ml of phosphate buffer (pH 6.3) was added to all the above samples after cooling. UV-Visible spectrophotometer (Analab) was used to measure the absorbance at 255 nm. The control represents 100% protein denaturation. The percentage inhibition of protein denaturation was calculated by the following formula:
Percentage inhibition = 100 – [{(optical density of test solution – optical density of product control)/ optical density of test control}/× 100].
Egg albumin denaturation method: 0.2 ml of fresh hen’s egg albumin, 2.8 ml of phosphate buffered saline (pH 6.4) and test solution of different concentrations (2, 5, 10, 20, 30 and 60 μg/ml) or standard drug diclofenac sodium (0.05 ml) of different concentrations (2, 5, 10, 20, 30 and 60 μg/ml) were mixed to form a reaction mixture of 5 ml. Double distilled water of same volume served as control. The samples were incubated at 37±2 ºC in a BOD incubator for 15 min followed by heating at 70 ºC for 5 min. UV-Visible spectrophotometer (Analab) was used to measure the absorbance at 255 nm. The percentage inhibition of protein denaturation was calculated by the following formula:
Percentage inhibition = 100 × [absorbance of test sample/absorbance of control – 1]
In vivo anti-arthritic activity
In vivo anti-arthritic activity of BFV was evaluated using collagenase induced osteoarthritis model [19].
Collagenase induced arthritis model: Induction of osteoarthritis was done in adult Wistar male albino rats (120±10 gm) by intraarticular injection of 20 μl bacterial collagenase (5 CDU) solution in the right knee joint. Same amount of saline was injected in the left knee joint. After treatment of 14 days, animals were sacrificed and serum pro inflammatory cytokines were measured.
Treatment schedule: Animals were randomised in 4 groups (n=6). Group 1: Sham control, Group 2: Osteoarthritic control, Group 3: Standard drug, Indomethacin treated (0.25 mg.kg-1, p.o. × 5 days, alternatively), Group 4: BFV treated (0.3 mg.kg-1, i.p. × 14 days).
Measurement of serum cytokines: Serum pro inflammatory cytokines TNF-α, IL-1β and IL-17 were measured using ELISA kits (R & D Systems, USA).
In vitro anti-inflammatory activity
In vitro anti-inflammatory activity of BFV was evaluated using RBC membrane stabilization method [20].
RBC membrane stabilization method: Goat red blood cells were used for the study of membrane stability. Goat blood was collected and mixed with 3.8% tri-sodium citrate solution and centrifuged at 3000 rpm. After washing the packed cells in isosaline, 10% suspension was made. 4.5 ml reaction mixture was prepared with 0.5 ml of 10% RBC suspension, 1 ml of phosphate buffer (pH 7.4), 2 ml of hyposaline (0.25% NaCl) and 1 ml of test solution of different concentrations (2, 5, 10, 20, 30 and 60 μg/ml). The samples were then incubated at 37 ºC for 30 min followed by centrifugation for 20 min at 3000 rpm. Supernatant solution was tested for haemoglobin content at 560 nm using a UV-Visible spectrophotometer (Analab).
In vivo anti-inflammatory activity
In vivo anti-inflammatory activity of BFV was evaluated using histamine induced paw edema model [21] and formalin induced paw edema model [22].
Histamine induced paw edema model: Histamine induced paw edema was analyzed in mice model. Mice were randomised into three groups (n=6). Gr.1: Histamine control, Gr.2: Standard drug, Indomethacin treated (10 mg.kg-1, i.p.), Gr.3: BFV treated (1.25 mg.kg-1, i.p.). The initial volume of right hind paw of mice was measured using digital caliper (Mitutoyo, Japan). Then, 0.02 ml of 1% histamine was injected subcutaneously into the subplantar region of right hind paw. The volume of right hind paw was measured at 1, 2, 3 hour after histamine injection for determining the volume of paw edema. Standard drug, Indomethacin and BFV were given one hour before histamine injection.
Formalin induced paw edema model: Formalin induced paw edema was analyzed in mice model. Mice were randomised into three groups (n=6). Gr.1: Formalin control, Gr.2: Standard drug, Indomethacin treated (10 mg.kg-1, i.p.), Gr.3: BFV treated (1.25 mg.kg-1, i.p.). The initial volume of right hind paw of mice was measured using digital caliper (Mitutoyo, Japan). Then, 0.02 ml of 2% formalin was injected subcutaneously into the subplantar region of right hind paw. The volume of right hind paw was measured at 1, 2, 3 hour after formalin injection for determining the volume of paw edema. Standard drug, Indomethacin and BFV were given one hour before formalin injection.
Statistical analysis
Data expressed as mean ± SEM (n=6). One way ANOVA was carried out for determination of significant differences between groups. *p< 0.05 was considered to be statistically significant (Gr.1 vs Gr.2) and **p< 0.05 was considered to be statistically significant (Gr.2 vs Gr.3/Gr.4).
Results
In vitro anti-arthritic activityEffect of BFV on bovine serum albumin (BSA) denaturation: BFV at a concentration of 2, 5, 10, 20 and 30 μg/ml showed inhibition of BSA denaturation by 3.06%, 4.36%, 4.97%, 9.39% and 20.86% respectively whereas standard drug, diclofenac sodium at a concentration of 2, 5, 10, 20 and 30 μg/ml showed inhibition of BSA denaturation by 2.92%, 5.69%, 9.79%, 13.05% and 20.29% respectively (Figure 1).
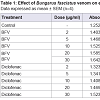
Effect of BFV on egg albumin denaturation: BFV at a concentration of 2, 5, 10, 20 and 30 μg/ml showed inhibition of egg albumin denaturation by 12.06%, 17.25%, 21.81%, 26.59% and 31.15% respectively whereas standard drug, diclofenac sodium at a concentration of 2, 5, 10, 20 and 30 μg/ml showed inhibition of egg albumin denaturation by 5.67%, 7.11%, 12.46%, 17.33% and 21.65% respectively (Table 1).
Figure 2: Effect of Bungarus fasciatus venom on serum proinflammatory cytokines TNF-α, IL-1β and IL-17 levels in FCA induced rats. Data expressed as mean ± SEM (n=6). One-way ANOVA was done for statistical analysis and *p<0.05 was considered as significant, (Gr.1 vs Gr.2) and **p<0.05 was considered as significant, (Gr.2 vs Gr.3/Gr.4) where Gr.1-Sham control, Gr.2- Arthritis control, Gr.3- Standard drug (Indomethacin) treated, Gr.4- BFV (0.3 mg.kg-1) treated.
Effect of BFV on RBC membrane stabilization: BFV at a concentration of 2, 5, 10, 20 and 30 μg/ml showed stabilization of RBC in hypotonic solution by 25.98%, 32.66%, 40.43%, 56.40 % and 60.29% respectively whereas standard drug, diclofenac sodium at a concentration of 2, 5, 10, 20 and 30 μg/ml showed stabilization of RBC in hypotonic solution by 22.67%, 29.84%, 45.52%, 52.59% and 56.66% respectively (Figure 3).
In vivo anti-inflammatory activity
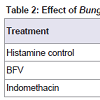
Effect of BFV on histamine induced paw edema: BFV showed significant reduction of histamine induced paw edema by 65.85% whereas standard drug, Indomethacin showed significant reduction of histamine induced paw edema by 70.73% when compared to histamine control group (Table 2).
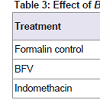
Effect of BFV on formalin induced paw edema: BFV showed significant reduction of formalin induced paw edema by 34.23% whereas standard drug, Indomethacin showed significant reduction of formalin induced paw edema by 44.97% when compared to formalin control group (Table 3).
Discussion
Rheumatoid arthritis, an autoimmune disease, involves denaturation of proteins and hence production of auto-antigens. Heat induced denaturation of BSA involves presentation of antigens that are related to diseases such as rheumatoid arthritis [23]. Denaturation of BSA was inhibited by BFV which suggested that BFV might prevent denaturation of protein in rheumatoid arthritis and hence a potential anti-arthritic agent. Heat induced denaturation of egg albumin was inhibited by BFV which again suggested that BFV might prevent protein denaturation in rheumatoid arthritis and hence a potential anti-arthritic agent.Due to infiltration of T-lymphocytes and macrophages into the synovial joint as well as their presence in peripheral blood, high levels of pro-inflammatory cytokines like TNF-α, IL-1β and IL-17 were found in the serum of arthritic patients [24]. Activated macrophages release TNF-α which in turn induces the release of IL-1β. Activated T-cells release IL-17 which in turn act as a stimulator of the release of TNF-α and IL-1β from macrophages [25]. BFV significantly reduced the levels of pro-inflammatory cytokines TNF-α, IL-1β and IL-17, this indicated that BFV might restore the balance of cytokines and hence could act as a potential anti-arthritic agent.
It is known that membrane of RBC is structurally equivalent to the lysosomal membrane. So any substance protecting RBC membrane can be predicted as a stabilizer of lysosomal membrane. Extracellular release of lysosomal content from neutrophil causes tissue damage and inflammation in arthritis [26]. RBC membrane lyses due to different injuries and causes haemolysis and oxidation of haemoglobin. Such type of damage of RBC further causes secondary damage through free radical induced lipid peroxidation as well as release of inflammatory mediators like phospholipases [11]. According to our result BFV could actively protect RBC membrane lysis and hence could be predicted as a potential anti- nflammatory agent.
Histamine induced inflammation is a good model for studying paw edema as histamine is a potent vasodilator, increases vascular permeability and can attract neutrophils at the target site. This results in increased blood flow and plasma exudates leak into the inflamed area, ultimately leading to redness and swelling [27]. Histamine induced paw edema was significantly reduced by BFV which suggested that BFV might interfere with the release or synthesis of this inflammatory mediator. Formalin induced inflammation is due to cell damage that result in the release of other inflammatory mediators like histamine, serotonin, bradykinin and prostaglandins [22]. This ultimately leads to redness, swelling and edema. Formalin induced paw edema was significantly reduced by BFV which suggested that BFV might interfere with the release or synthesis of these inflammatory mediators.
Conclusion
It may be concluded that Bungarus fasciatus venom possesses significant in vitro and in vivo anti-arthritic and anti-inflammatory activities. Further studies are warranted on the active venom constituents involved in this process.Acknowledgements
This work was sponsored by University Grants Commission (UGC), New Delhi. [Ref.No:F.No.42- 536/2013(SR)dt. 20.03.13].References
- Riegsecker S, Wiczynski D, Kalpan MJ, Ahmed S (2013) Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci 93: 307-312.
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, et al. (2000) Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 133: 635-646.
- Hedbom E, Hauselmann HJ (2002) Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci 59: 45-53.
- Lees P (2003) Pharmacology of drugs used to treat osteoarthritis in veterinary practice. Inflammopharmacology 11: 385-399.
- Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M (1997) Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 24: 365-371.
- Fiorito S, Magrini L, Adrey J, Mailhe D, Brouty-Boye D (2005) Inflammatory status and cartilage regenerative potential of synovial fibroblasts from patients with osteoarthritis and chondropathy. Rheumatology 44: 164-171.
- Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39: 237-246.
- Schulte E, Fisseler-Eckhoff A, Müller KM (1994) Differential diagnosis of synovitis. Correlation of arthroscopic-biopsy to clinical findings. Pathologe 15: 22-27.
- Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423: 356-361.
- McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7: 429-442.
- Gaffo A, Saag KG, Curtis JR (2006) Treatment of rheumatoid arthritis. Am J Health Sys Pharm 63: 2451-2465.
- Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, et al. (1996) Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis: A prospective observational cohort study. Arch Intern Med 156: 1530-1536.
- Emmanuel JH, Montgomery RD (1971) Gastric ulcer and the anti-arthritic drugs. Postgrad Med J 47: 227-232.
- Pal SK, Gomes A, Dasgupta SC, Gomes A (2002) Snake venom as therapeutic agents: from toxins to drug development. Indian J Exp Biol 40: 1353-1358.
- Gomes A, Datta P, Das T, Biswas AK, Gomes A (2014) Anti arthritic and anti inflammatory activity of a cytotoxic protein NN-32 from Indian spectacle cobra (Naja naja) venom in male albino rats. Toxicon 90: 106-110.
- Bhattacharya S, Das T, Biswas A, Gomes A, Dungdung SR, et al. (2013) A cytotoxic protein (BF-CT1) purified from Bungarus fasciatus venom acts through apoptosis, modulation of PI3K/AKT, MAPKinase pathway and cell cycle regulation. Toxicon 74: 138-150.
- Wang Y, Hong J, Liu X, Yang H, Liu R, et al. (2008) Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS One 3: e3217.
- Rahman H, Eswaraiah MC, Dutta AM (2015) In-vitro anti-inflammatory and anti-arthritic activity of Oryza sativa var. joha rice (an aromatic indigenous rice of Assam). American-Eurasian J Agric Environ Sci 15: 115-121.
- Van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB (1989) Development of osteoarthritic lesions in mice by "metabolic" and "mechanical" alterations in the knee joints. Am J Pathol 135: 1001-1014.
- Lavanya R, Maheshwari SU, Harish G, Raj JB, Kamali S, et al. (2010) Investigation of in vitro anti-inflammatory, anti-platelet and anti-arthritic activities in the leaves of Anisomeles malabarica Linn. RJPBCS 1: 745-752.
- Gupta M, Mazumder UK, Gomathi P, Selvan VT (2006) Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Complement Altern Med 6: 36.
- Dimo T, Fotio AL, Nguelefack TB, Asongalem EA, Kamtchouing P (2006) Antiinflammatory activity of leaf extracts of Kalanchoe crenata Andr. Indian J Pharmacol 38: 115-119.
- Williams LA, O’Connar A, Latore L, Dennis O, Ringer S, et al. (2008) The in vitro anti-denaturation effects induced by natural products and nonsteroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med J 57: 327-331.
- Hussein MR, Fathi NA, El-Din AME, Hassan HI, Abdullah F, et al. (2008) Alterations of the CD4+, CD8+ T cell subsets, interleukins-1β, IL-10, IL-17, tumor necrosis factor-α and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: preliminary observations. Pathol Oncol Res 14: 321-328.
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, et al. (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J Immunol 160: 3513-3521.
- Shendkar AK, Chaudhari SG, Shendkar YK (2014) In vitro antiarthritic activity of Withania coagulans dunal fruits. IAJPR 4: 915-924.
- Ekeuku SO, Okechukwu PN (2014) Paw edemaand bronchoconstriction induced by mediators: how they were inhibited in vivo by partially purified extract from dichloromethane leaf extract of Labisia pumila. Middle-East J Sci Res 22: 1114-1121.