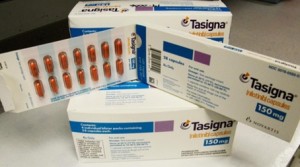
Parkinson’s disease (PD) is typically considered a chronic, progressive neurodegenerative movement disorder. The most obvious symptoms are shaking, rigidity, slowness of movement, and difficulty with walking. When this happens a drug nilotinib (Tasigna) found that movement and mental function improved in all of the people who completed the six-month trial in research, one woman regained the ability to feed herself, one man was able to stop using a walker, and three previously nonverbal patients began speaking again. If the drug’s effectiveness is confirmed in larger, placebo-controlled studies, nilotinib could become the first treatment to interrupt a process that kills brain cells in Parkinson’s and other neurodegenerative diseases, including Alzheimer’s.
The study found that levels of toxic proteins in blood and spinal fluid decreased once patients began taking nilotinib. Also, tests showed that the symptoms of Parkinson’s, including tremor and “freezing” decreased. And during the study, patients were able to use lower doses of Parkinson’s drugs, suggesting that the brain cells that produce dopamine were working better, but after taking of nilotinib dose leads to other worse symptoms in the patients, like heart rhythms. Most of the Parkinson’s patients are generally older and often have other conditions that can raise their risk of heart problems. With nilotinib already on the market, some patients and doctors might be tempted to try it, but some of the doctors cautioned against that. This drug has already been tested and proven safe in clinical trials for leukemia, which could expedite its use in PD. So, we should not rush to conclusions.